

Background: Behçet’s disease (BD) is a multisystemic relapsing vasculitis of unknown etiology. Ocular inflammation, typified by uveitis, was reported in about 50% of BD patients. Polymorphonuclear neutrophil (PMN) hyperactivation, characterized by the overproduction of neutrophil extracellular traps (NETs) and proinflammatory cytokines, is increasingly implicated in BD [1]. Focusing on immunometabolism, we have comprehensively analyzed serum metabolomics and peripheral immunocyte transcriptomics in BD and healthy controls (HCs). Our previous findings revealed a dysregulation of mevalonate pathway in BD-PMN, resulting in a significant upregulation of the metabolite farnesyl pyrophosphate (FPP), and FPP levels in both BD-PMN and serum were positively correlated with disease activity [2].
Objectives: This study aims to investigate the underlying mechanism of FPP-induced BD-PMN hyperactivation and to offer an immunometabolic perspective for BD pathogenesis.
Methods: We measured the production of pro-inflammatory cytokines, NETs, and calcium flux in FPP-stimulated PMN by qRT-PCR, immunofluorescence, and flow cytometry, respectively. Experimental autoimmune uveitis (EAU) is widely used in studies of BD. To confirm the critical role of FPP in PMN activation and BD pathogenesis, we induced EAU in myeloid cell-specific FPP synthetase (FPPS) knockout (FPPS flox/flox LysMcre) mice and FPPS flox/flox control mice by immunization with IRBP (peptide 1-20) and PTX, and evaluated morbidity, severity and PMN activation. Mechanistically, we comprehensively analyzed the transcriptomics of ruthenium red (RR)-sensitive calcium channels in BD-PMN and validated with western blotting. In addition, HC-PMN was incubated with active BD serum, or proinflammatory cytokines upregulated in BD, including TNF-α, IL-6, and IFN-γ, to identify the key cytokine promoting TRPM2 expression. Furthermore, the expression level of TRPM2 in PMN from BD patients was measured pre- and post-therapy with TNF-α inhibitors.
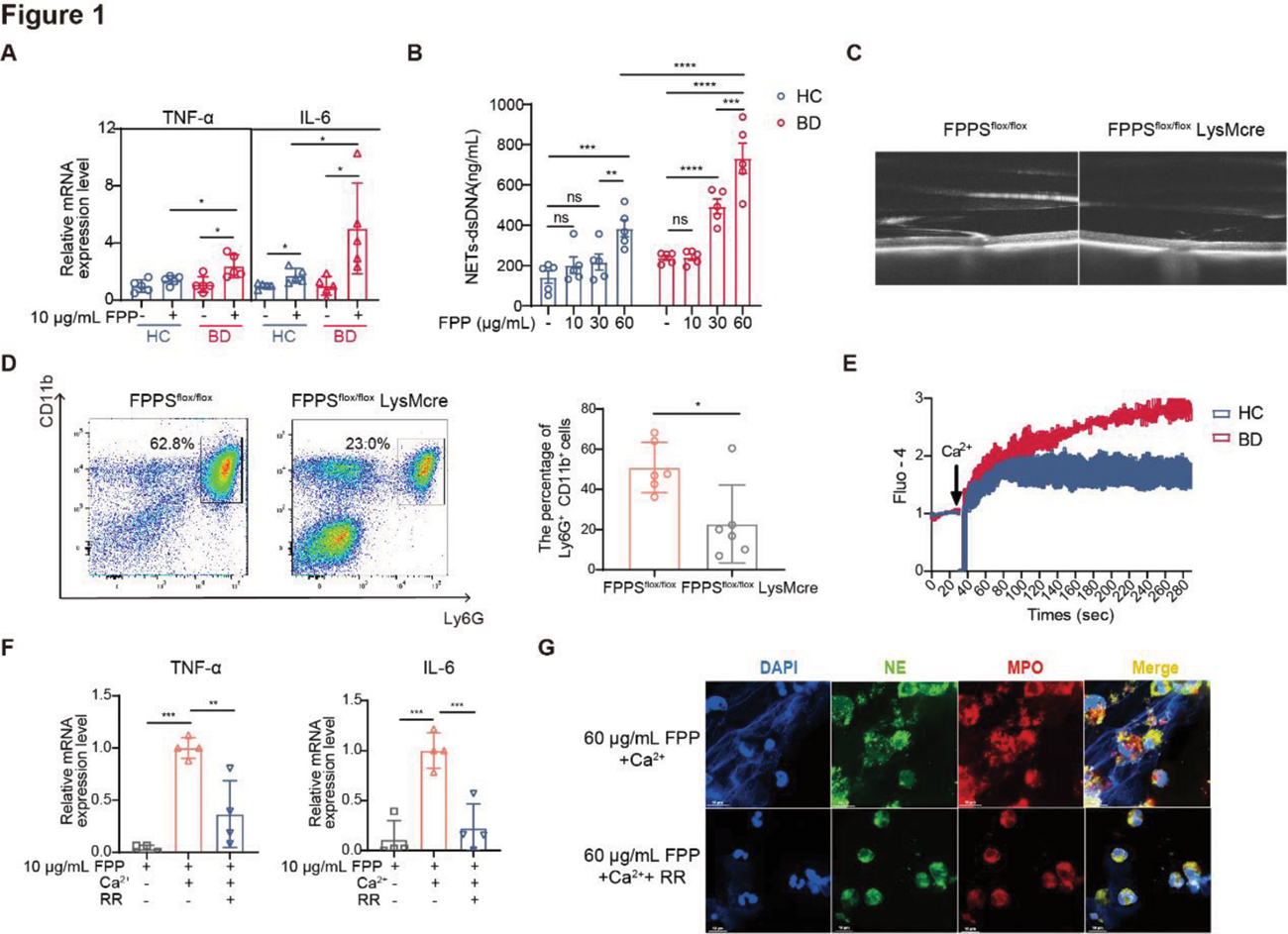
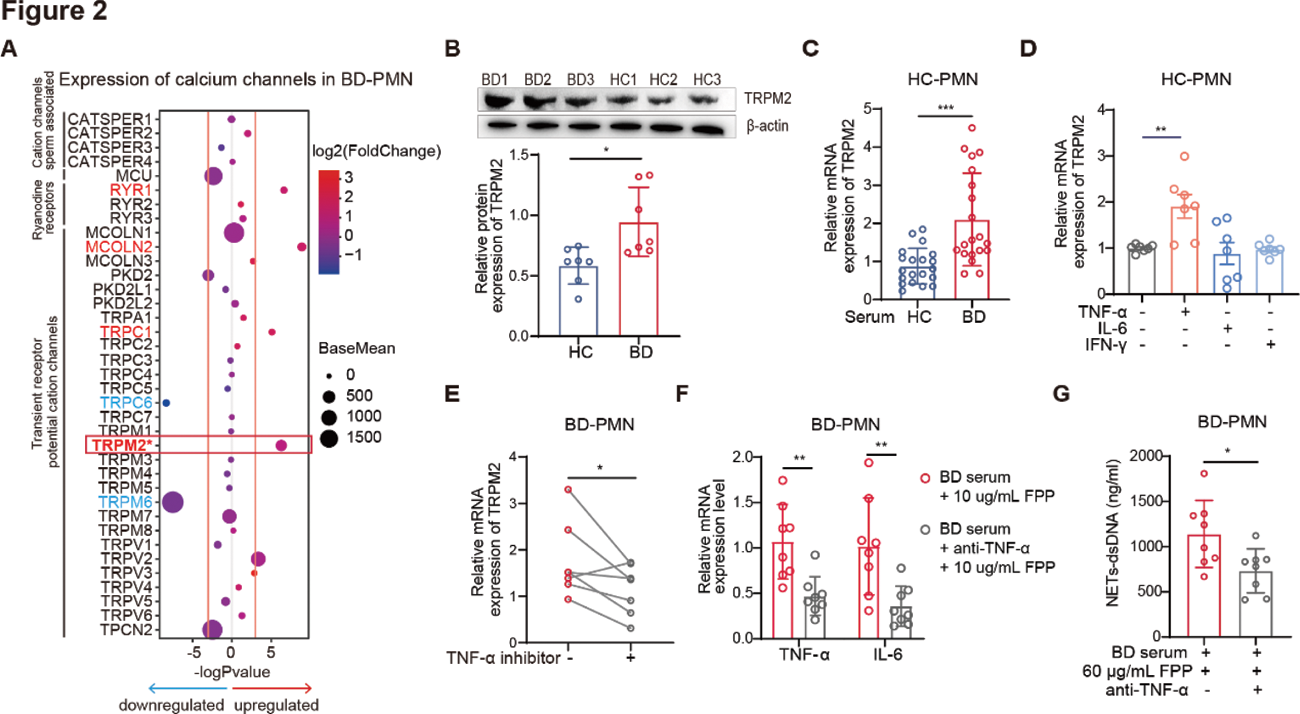
Results: FPP specifically promoted PMN to produce proinflammatory cytokines and NETs in a dose-dependent manner, with stronger responses in BD-PMN (Figure 1A-B). Notably, FPPS flox/flox LysMcre mice showed lower EAU incidence (57.1% vs 27.4%), milder severity, and reduced neutrophil activation (decreased Ly6G + CD11b + % and increased CD62L expression, p=0.0115) in vivo , compared to control mice (Figure 1C-D). Additionally, FPP induced stronger calcium flux in BD-PMN (Figure 1E), and removal of Ca 2+ or addition of RR, a transient receptor potential (TRP) calcium channel inhibitor, significantly reduced FPP-induced PMN activation (Figure 1F-G), suggesting a TRP-dependent mechanism. To further elucidate the hyperresponsiveness of BD-PMN to FPP, we comprehensively analyzed the transcriptomics of RR-sensitive calcium channels and identified that TRPM2 was upregulated in BD-PMN (Figure 2A-B). Of note, active BD serum and TNF-α (but not IL-6 or IFN-γ) potently upregulated TRPM2 expression in HC-PMN (Figure 2C-D). Furthermore, therapy with TNF-α inhibitors effectively downregulated TRPM2 expression in BD-PMN in vivo (Figure 2E), and TNF-α neutralizing inhibition mitigated FPP-induced BD-PMN hyperactivation in vitro , as evidenced by a significant decrease in the production of both proinflammatory cytokines and NETs (Figure 2F-G).
Conclusion: From an immunometabolism perspective, our study highlights that FPP promotes BD-PMN hyperactivation via TRPM2, providing insight into the potential of targeting FPP in treating BD. It also sheds light on a novel therapeutic mechanism of TNF-α inhibitors in BD.
REFERENCES: [1] Yu X, Li L, Zhang M, et.al. Transcriptional analysis of neutrophils from patients with Behçet’s disease reveals activation and chemotaxis of neutrophils. Clin Immunol. 2022 Dec;245:109161.
[2] Zhang M, Kang N, Yu X, et.al. Aberrant Mevalonate Metabolite Farnesyl Pyrophosphate-Induced Neutrophil Hyperactivation in Behçet’s Disease Pathogenesis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9).
Acknowledgements: We thank the health professional staff from the Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital and appreciate for the participation of all the patients and healthy volunteers in this study.
Disclosure of Interests: None declared.