

Background: Glucocorticoids (GC) act intracellularly to treat rheumatic diseases but can induce side effects such as osteopenia and insulin resistance. 11β-hydroxysteroid dehydrogenase type 1 (HSD-1) is a key tissue-specific intracellular modulator of GC action in bone, liver, adipose, brain, and other tissues. HSD-1 deficiency or inhibition has been consistently associated with reduced GC side effects in mouse and human. Mouse results suggest that HSD-1 deficiency shifts dose-response relationships upward for GC-induced immune suppressive effects. SPI-62, a potent HSD-1 inhibitor, prevented GC-associated insulin resistance, weight gain, hyperphagia, muscle atrophy, adiposity, and dermal thinning in mouse.
Objectives: To learn how SPI-62 alters prednisolone (PSL) efficacy and side effects in patients with PMR. We predicted that desired immunosuppressive effects could be achieved without tissue-specific side effects by adjusting PSL dose to compensate for immune effects of HSD-1 inhibition.
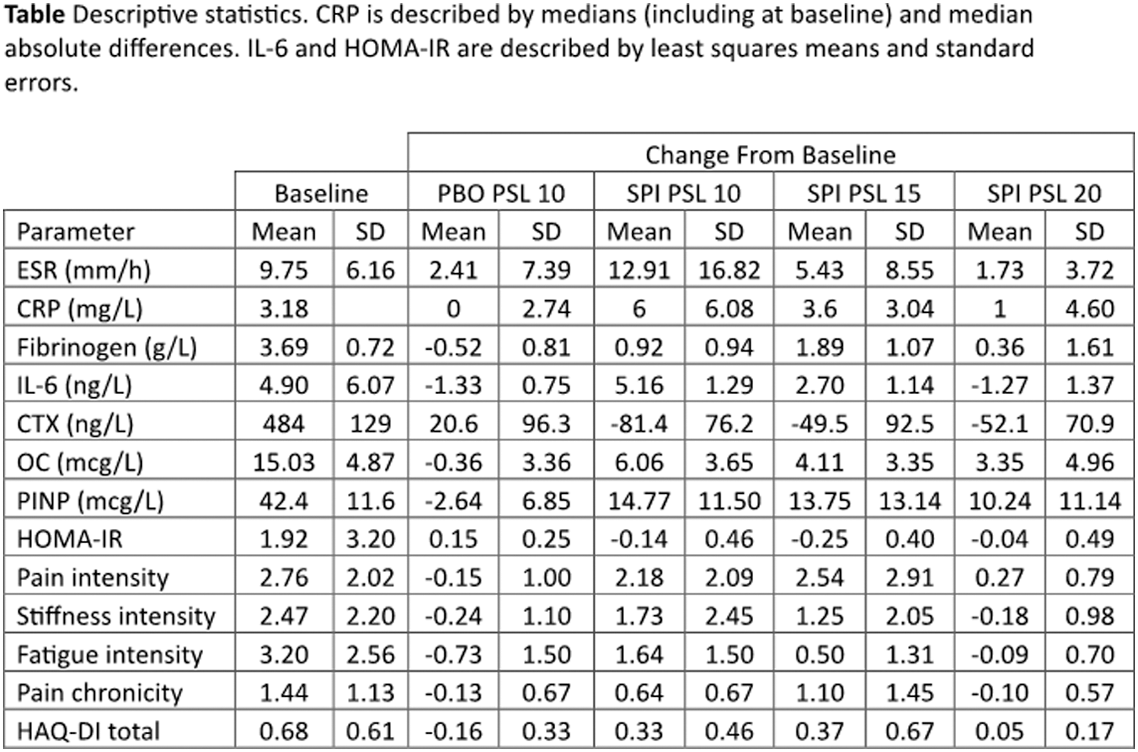
Methods: Patients with PMR, taking PSL 10 mg/day, and not in need of dose increase were included. Participants continued PSL without dose reduction for 4 weeks and also received SPI-62 6 mg daily or matching placebo (PBO), each for 2 weeks. In sequential cohorts, during SPI-62 treatment PSL daily dose was maintained at 10 mg (SPI PSL 10), adjusted to 15 mg (SPI PSL 15), or adjusted to 20 mg (SPI PSL 20). PBO for additional PSL was administered with PBO for SPI-62 (PBO PSL 10). If the investigator considered the participant’s PMR worsened, adverse event of PMR aggravated was recorded. Relapse was defined by investigator’s decision to increase PSL dose. Participants completed numeric rating scales (NRS) of pain, stiffness, and fatigue intensity, and pain chronicity, daily and HAQ-Disability Index (HAQ-DI) at trial visits. The maximum value reported on each NRS during a week was used for analysis. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fibrinogen, interleukin 6 (IL-6), osteocalcin (OC), procollagen type I N-propeptide (PINP), and C-terminal telopeptide of type I collagen (CTX) were measured pre-dose at Day 1, Week 2, and Week 4. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin. Continuous parameters were characterized by effect sizes on change from baseline to enable comparison of SPI-62 impact across parameters on a consistent metric. Effect sizes were based on means except CRP (median), IL-6, and HOMA-IR (least squares mean). Hepatic HSD-1 inhibition was monitored as the ratio (tetrahydrocortisol + allotetrahydrocortisol)/ tetrahydrocortisone in urine.
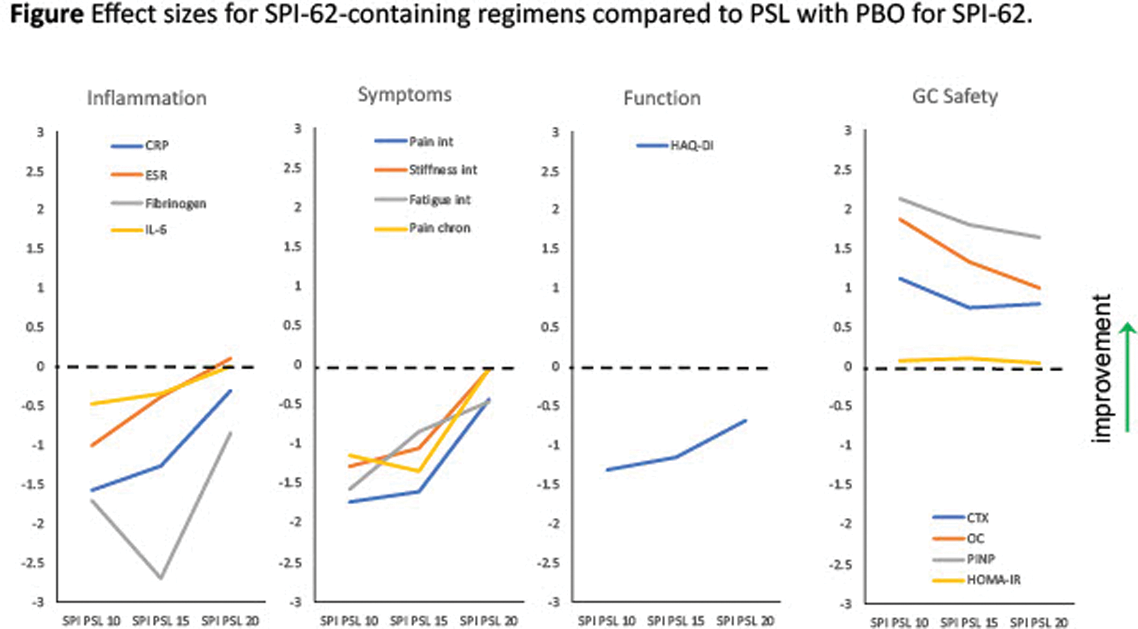
Results: In participants who received PBO PSL 10 (n = 38), SPI PSL 10 (n = 13), SPI PSL 15 (n = 12) and SPI PSL 20 (n = 11), PMR aggravated was reported for 0, 6, 1, and 2, and relapse experienced by 0, 5, 0, and 0. Effect sizes are presented in the Figure 1. Descriptive statistics are presented in the Table 1. SPI-62 achieved 94.7 ± 4.6% hepatic HSD-1 inhibition.
Conclusion: SPI-62 achieved full HSD-1 inhibition. SPI PSL 10 attenuated PMR disease control, which was partly restored by SPI PSL 15 and further by SPI PSL 20. Relapses, ESR, IL-6, stiffness intensity, and pain chronicity were numerically the same with SPI PSL 20 as PBO PSL 10. With appropriate PSL dose adjustment, SPI-62-was associated with large improvements on OC, PINP, and CTX, consistent with an anti-osteopenic effect. SPI-62 was also associated with small but consistent improvements on HOMA-IR. SPI PSL 20, compared to PBO PSL 10, was associated with similar effects on several measures of PMR efficacy and benefits on biomarkers related to GC side effects. That shows, for the first time, differential modulation of GC efficacy and toxicity by a HSD-1 inhibitor in patients with a rheumatic disease. Additional PSL doses are being tested to optimize the therapeutic profile of PSL co-administration with SPI-62 in PMR.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: David A Katz Sparrow, Sparrow, Frank Buttgereit AbbVie, Sanofi, Pfizer, Sparrow, AbbVie, Sanofi, Gruenenthal, Horizon, AbbVie, Sanofie, Horizon, Andrea Everding: None declared, Ioana Andreica AbbVie, Chugai, Gilead, Lilly, MSD, Novartis, Pfizer, SOBI, UCB, Amgen, BI, Chugai Pharma, Galapagos, Lilly, Novartis, Pfizer, SOBI, UCB, Takeda, Lilly, Herbert L Kellner: None declared, Florian Schuch AbbVie, Galapagos, Lilly, Novartis, AbbVie, Galapagos, Lilly, Novartis, AbbVie, Galapagos, Lilly, Novartis, Tonya K Marmon Sparrow, Frank S Czerwiec Sparrow, Sparrow, Ketan Desai Sparrow.