

Background: Both the ACR[1] and EULAR[2] guidelines endorse the use of colchicine or NSAIDs for gout flare prophylaxis when initiating urate-lowering therapy for gout care; however, a recent UK study[3] found 1.6- and 1.9-fold higher risk of myocardial infarction (MI) associated with colchicine and NSAID prophylaxis (compared to no active comparator), respectively, when starting allopurinol for gout. These findings are surprising and concerning,[4] as gout patients are already at high risk of major adverse cardiovascular (CV) events (MACE).[5] Furthermore, this increased risk associated with colchicine contradicts the randomized trial results[6] and FDA approval for its cardiovascular benefit.
Objectives: To emulate target trials to determine the risk of MI and MACE associated with colchicine or NSAID prophylaxis when initiating allopurinol for gout care.
Methods: Using a Canadian general population database, we designed and conducted our primary target trial emulation (TTE) to compare colchicine vs. NSAID (active comparator) for the risks of MI and MACE among patients with gout starting allopurinol for gout care. Randomization was emulated using propensity score matching based on 60 pre-exposure MI/MACE-related covariates, including demographics, comorbidities, medication use, and healthcare utilization. Our primary TTE followed individuals for 3 months or until the first occurrence of MI (or MACE) or end of colchicine or NSAID treatment, whichever came first (3 month-as treated [AT]). Sensitivity analyses followed individuals for 6 months or until the first occurrence of MI (or MACE) or end of colchicine or NSAID treatment (6 month-AT), and regardless of treatment cessation (intention-to-treat, ITT) for 3 and 6 months (3 month-ITT and 6 month-ITT, respectively). Our secondary TTEs compared 1) colchicine vs. no prophylaxis and 2) NSAID vs. no prophylaxis (i.e., no active comparator, as done in the recent UK study, 3 where the causal contrast was limited to 6 month-AT).
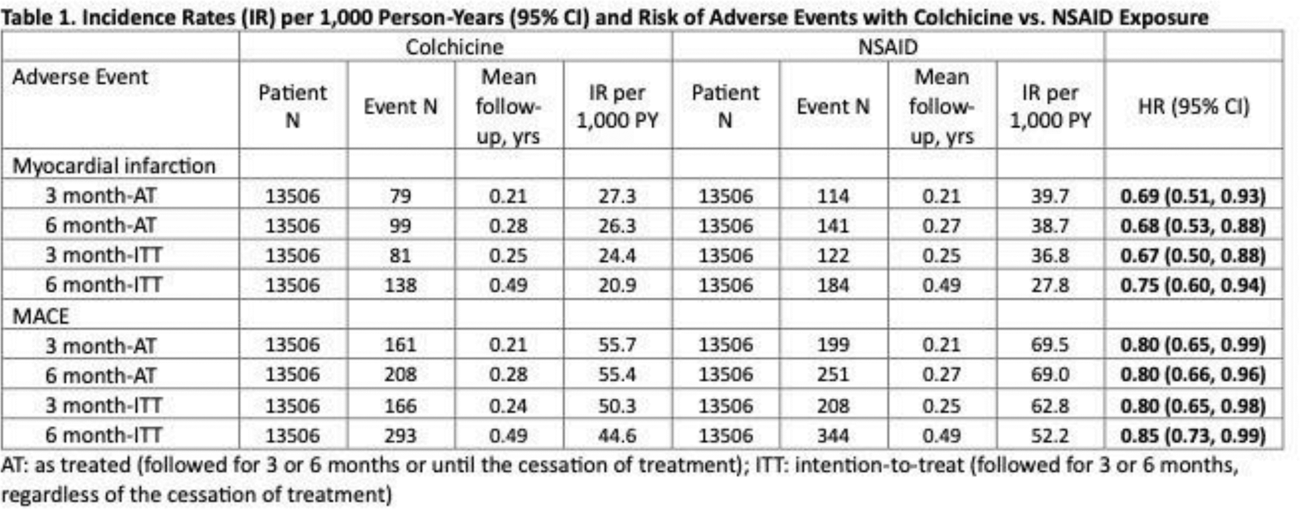
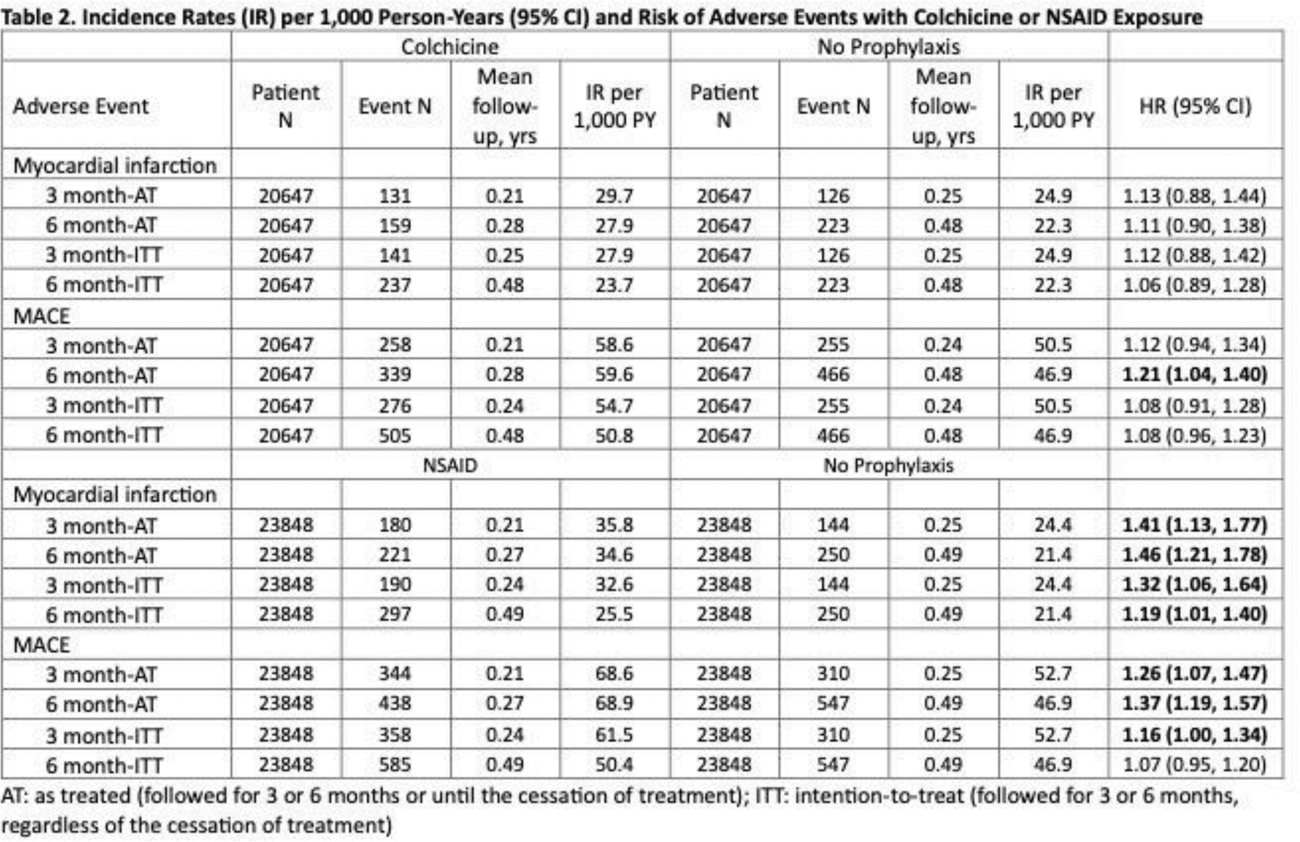
Results: In all emulated target trials, the baseline characteristics of patients (including CV diseases and CKD) in each treatment group were well-balanced after 1:1 propensity score matching (all standardized differences < 0.1). We identified 13,506 individuals who started allopurinol with colchicine prophylaxis and 13,506 individuals who started allopurinol with NSAID prophylaxis. After propensity score matching, the incidence rates for MI for the primary analysis were 27.3 per 1000 person-years (PYs) for colchicine and 39.7 per 1000 PY for NSAID, resulting in a hazard ratio (HR) of 0.69 (95% CI; 0.51, 0.93) ( Table 1 ). The incidence rates for MACE were 55.7 per 1000 PY for colchicine and 69.5 per 1000 PY for NSAID, resulting in a HR of 0.80 (95% CI; 0.65, 0.99) ( Table 1 ). These findings persisted in all sensitivity analyses with varying causal contrasts and durations ( Table 1 ). Colchicine was not associated with MI or MACE risk compared to no prophylaxis, except for MACE in the 6 month-AT analysis, the approach employed by the UK study 3 that resulted in a major imbalance in follow-up times (as the no prophylaxis group had no treatment to stop for 6 months , unlike colchicine group) ( Table 2 ). Conversely, in all TTEs, NSAIDs were associated with a higher risk of MI and MACE compared with no prophylaxis, most prominently in the 6 month-AT analysis (as expected for the same reason above) ( Table 2 ).
Conclusion: In these target trial analyses emulating pragmatic trials of gout patients starting allopurinol in the general population, colchicine prophylaxis was associated with a lower risk of MI and MACE than NSAID, whereas the risk was unaltered compared with no prophylaxis. These TTE results agree with the known and expected cardiovascular effects of colchicine and NSAID, supporting colchicine as the preferred flare prophylaxis, particularly among gout patients with CV disease or risk factors.
REFERENCES: [1] FitzGerald et al., PMID: 32391934.
[2] Richette et al., PMID: 27457514.
[3] Roddy et al., PMID: 37788904.
[4] Yokose et al.,
[5] Choi et al., PMID: 17698728.
[6] Nidorf et al., PMID: 32865380.
Acknowledgements: NIL.
Disclosure of Interests: Chio Yokose: None declared, Natalie McCormick: None declared, Na Lu: None declared, Abhishek Abhishek: None declared, Angelo Gaffo SOBI, PK Med, Yuqing Zhang: None declared, Hyon Choi Ani, LG, Horizon, Shanton, and Protalix, Horizon.