

Background: B-cell mediated autoimmune disease, such as systemic lupus erythematosus (SLE), can cause widespread inflammation and organ damages, and even life-threatening symptoms for some severe patients. Despite tremendous progress in the clinical management of SLE, many patients do not respond to the currently used treatments. Autoreactive B cells play a key role in the pathogenesis of autoimmune diseases like SLE. Recently, clinical cases suggested that CAR-T therapies showed potential to promote full clinical remission in SLE.
Objectives: To evaluate the safety and efficacy of relma-cel in patients with active SLE.
Methods: An open-label, single arm, multicenter study (NCT05765006) to evaluate the safety, efficacy, and cellular kinetics of relma-cel in patients with active SLE in China is currently ongoing. Following the lymphodepleting therapy of cyclophosphamide 250 mg/m 2 /day and fludarabine 25 mg/m 2 /day, patients were to be dosed with 25 or 50 or 75x10^6 CAR+ T cells. For efficacy, we assessed the changes of autoantibodies, complement components (C3), SELENA-SLEDAI scores and proteinuria from baseline. We also analyzed the composite endpoint of response rates assessed by SRI-4 and LLDAS. For safety, we reported any level of adverse event (AEs) and serious adverse events (SAEs), including the CRS (cytokine release syndrome) and neurotoxicity (NT). We monitored CAR-T cell kinetics by quantitative polymerase chain reaction (PCR) and flow cytometry.
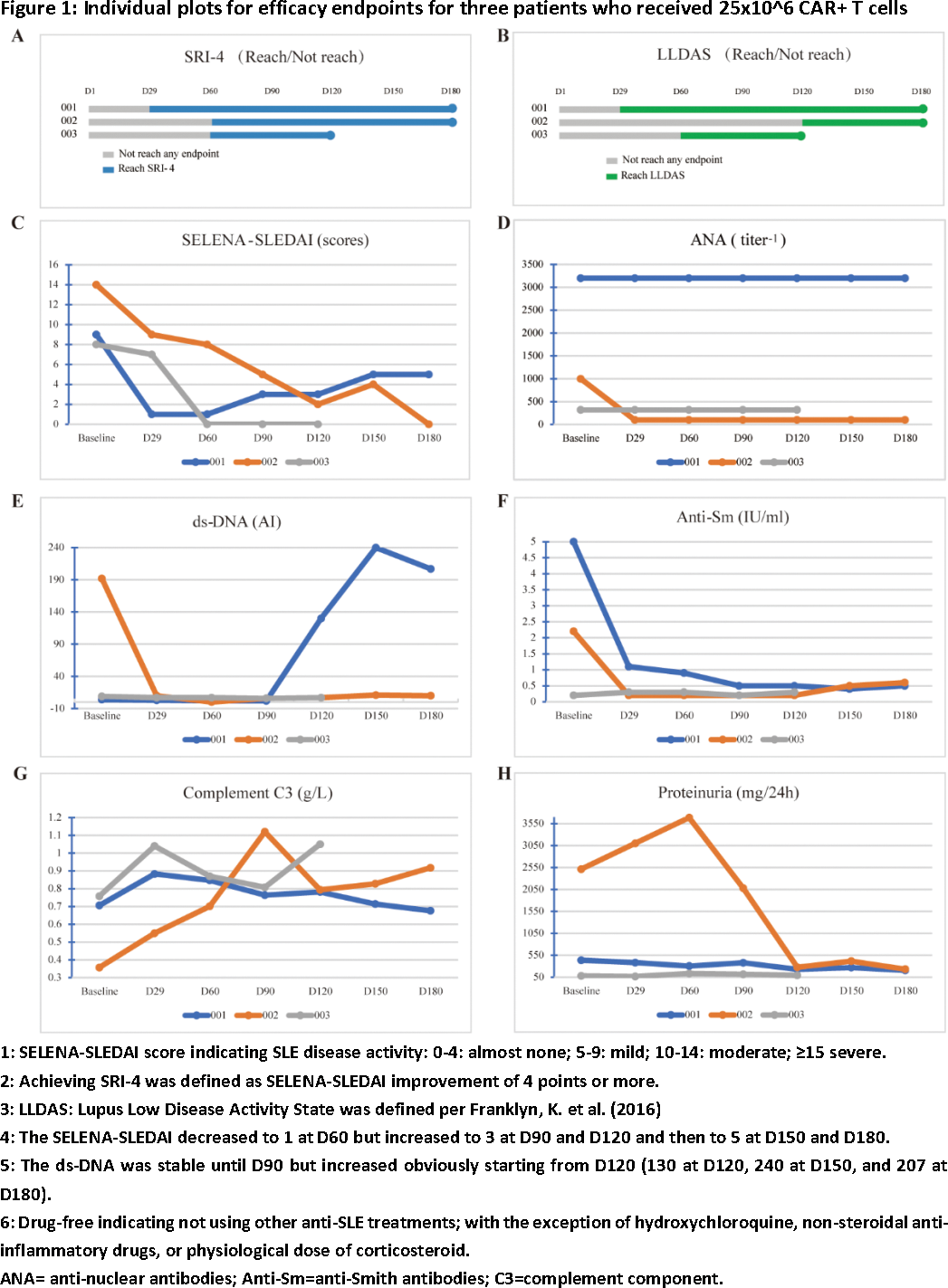
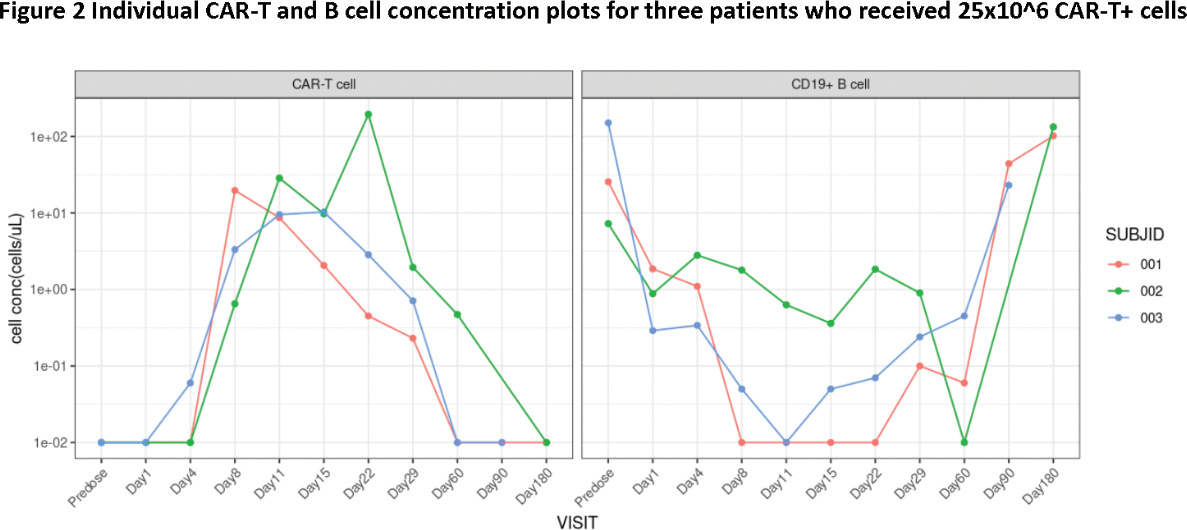
Results: As of 18 Dec 2023, three patients received 25x10^6 CAR+ T cells and were followed for at least 4 months. All patients were female, aged between 21 and 36 years. Two had a medical history of more than 10 years, and one had 1 year. All patients had multiorgan involvement, including 3 skin, 2 kidney, 2 hematology and 1 joint. All patients had active SLE at baseline, with SELENA-LEDAI between 8 and 14. Despite their young age, all patients previously exposed to high dose steroids and several immunosuppressive drugs (3 hydroxychloroquine [HCQ], 2 mycophenolate mofetil [MMF], 2 tacrolimus, 1 methotrexate [MTX]), and two patients received biological agents (2 telitacicept, 1 beliuzumab). Two patients received bridging therapies (1 corticosteroid and tacrolimus 1 corticosteroid, MMF and HCQ). After relma-cel infusion, Disease activity assessed by SELENA-SLEDAI decreased to 0 or 1. SRI-4 achieved in all patients. LLDAS achieved in two patients. Signs and symptoms of SLE continuously improved in all patients. Autoantibodies level of all patients declined, accompanied with elevated level of C3; only one patient’s anti-dsDNA level initially decreased but then increased from D120. Elevated proteinuria at baseline in two patients, and decreased to normal range after infusion. As of the data cut-off, all patients stayed drug-free, meaning not received any anti-SLE treatment after relma-cel infusion. Detailed data are shown in Figure 1. CRS occurred in two patients (one of grade 1 and one of grade 3). No NT reported. Cytopenia occurred in two patients. Infection, MAS and effusion were observed in one single patient. With steroid, anti-infection, and supportive treatment, the patient completely recovered around Day60. As of the data cut-off, all AEs occurred were resolved. PK data showed cellular expansion with a median peak concentration (C max ) of 19.72 cells/μL by flow cytometry and T max between 8-22 days post infusion. Complete B cell depletion were observed (shown as 0.01 cells/μL). The nadir of B concertation was observed from Day8-11 and then starting recovery at Day60. Majority (> 97%) of the recovered B cells were naïve B cells, consistent with pervious reports.
Conclusion: Previously known adverse events to be related to CD19-directed CAR-T cell therapy were observed as expected. Even with the lowest dose level of 25x10^6 CAR+ T cells, preliminary data from the first three patients suggested favorable profiles on safety, CAR-T cell expansion and B cell depletion, indicated promising efficacy of relma-cel in active SLE. Patients will be followed up further and more patients will be dosed with higher doses to observe long term safety and efficacy as well as to determine the potential target treatment dose.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Yu Hu: None declared, Mei Heng: None declared, Wei Xie: None declared, Chunli Mei: None declared, Danying Liao: None declared, Jinhui Shu: None declared, Zhaozhao Chen: None declared, Meilin Ma JW Therapeutics (Shanghai) Co. Ltd., Zisong Zhou JW Therapeutics (Shanghai) Co. Ltd., Weici Huang JW Therapeutics (Shanghai) Co. Ltd.