

Background: Patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapies have attenuated humoral vaccine responses and are prone to more severe infections. Assessing the persistence of cellular and humoral immunity following repeated SARS-CoV-2 vaccine doses and infection is important to evaluate the need for booster vaccine doses.
Objectives: To assess the cellular and humoral responses to 4 th (original) and 5 th (updated BA.1 or BA.4/5) SARS-CoV-2 vaccine doses and to COVID-19 following a 4 th vaccine dose (hybrid immunity) in IMID patients on tumour necrosis factor inhibitors (TNFi).
Methods: The ongoing observational Nor-vaC study includes IMID patients receiving multiple SARS-CoV-2 vaccines. In the present analyses, patients using TNFi who were eligible for a 5 th vaccine dose and/or undergoing COVID-19 following a 4 th vaccine dose, provided serum and peripheral blood mononuclear cells (PBMCs) 2-4 weeks and 4-11 months following infection, and before, 2-4 weeks, and 6 months after their 5 th vaccination. CD4 and CD8 T cell responses to SARS-CoV-2 spike, nucleocapsid and membrane peptides, and also IgG anti-spike antibodies, were analysed. T cell responses were measured by flow cytometry (≥0.01% increase in responding CD4 or CD8 cells compared to unstimulated cells).
Results: Between December 17 th , 2021, and June 20 th , 2023, 394 IMID patients (86 rheumatoid arthritis, 72 psoriatic arthritis, 97 spondyloarthritis, 86 Crohn’s disease, 53 ulcerative colitis) on TNFi received a 4 th and 5 th vaccine dose, and/or had COVID-19 following a 4 th vaccine dose. (Table 1). Infection induced a small increase in spike-specific CD4 T cell responses in individuals who had already received a 4 th dose (median (IQR) frequencies); four vaccine doses vs. hybrid immunity, 0.068% (0.034-0.16) vs. 0.11% (0.080-0.17), p=0.048). CD4 T cell responses after hybrid immunity waned over 4-11 months (0.035% (0.026-0.072), (hybrid immunity at 2-4 weeks vs. 4-11 months, p=0.0008). CD8 T cell responses following 4 th vaccine dose (0.040 % (0.010-0.14) remained stable after infection (0.054% (0.025-0.17) p=0.57), and also in the next 4-11 months (0.038% (0.013-0.17) p=0.63). (Figure 1) A 5 th vaccine dose restored CD4 T cell responses to the level seen 2-4 weeks after infection (0.089% (0.046-0.15), p=0.0093) compared with levels prior to 5 th vaccine dose. CD8 T cell responses to a 5 th vaccine dose did not significantly differ from those post-infection (0.057% (0.016-0.21) p=0.63). After infection, 32/36 (89%) patients had nucleocapsid-specific CD4 T cell responses and 23/36 (64%) had membrane-specific CD4 T cell responses. Similarly, 30/36 (83%) and 22/36 (61%) patients had nucleocapsid- and membrane-specific CD8 T cell responses. These responses decreased over time and were not boosted by vaccination. Patients with hybrid immunity had higher IgG anti-spike antibody levels than patients receiving only 4th dose (median 23159 IU/mL (IQR 14588-44529) vs 7242 (3220-17259) p<0.001. This humoral response waned prior to the 5 th dose but increased following the 5 th vaccine dose, median 9109 (3603-19839) vs. 31198 (13976-51224), p<0.001).
Conclusion: CD8 T cell responses after four immunisations showed no significant benefit of further boosting, suggesting long-lived cellular responses that could be clinically protective. CD4 T cell and humoral responses wane following both four vaccinations and infection but are restored by vaccine boosters, indicating that also IMID patients with hybrid immunity could benefit from a booster vaccine to maintain high levels of circulating antibodies and spike-specific T cells.
Table 1. Patient characteristics
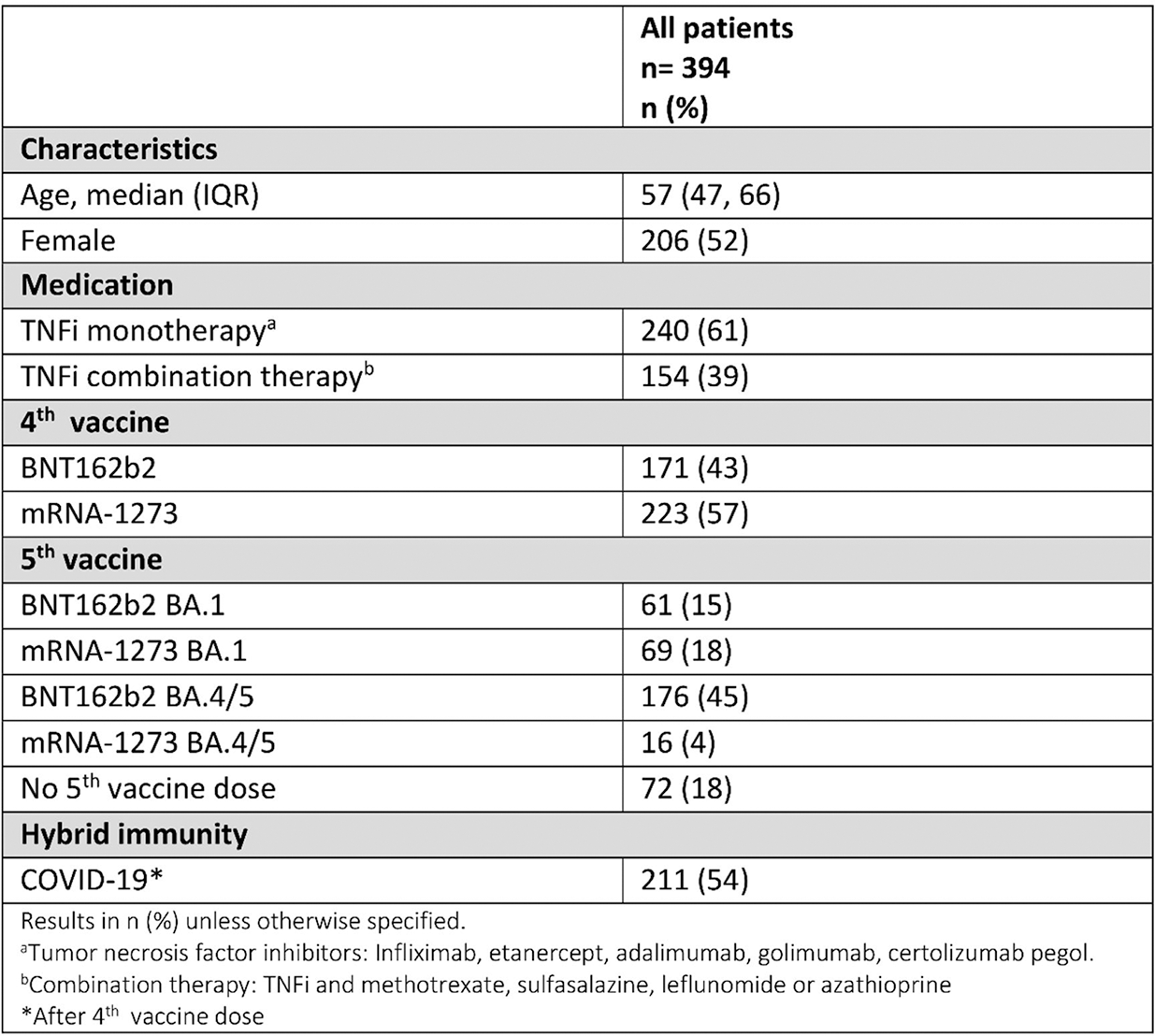
CD4 T cells and anti-spike antibody levels following a 4 th vaccine dose are boosted by infection (4 th dose hybrid), with subsequent waning prior to a 5 th vaccine dose before increasing after a 5 th dose, in contrast to stable CD8 T-cell responses.
REFERENCES: NIL.
Acknowledgements: We thank the patients who have participated in the Nor-vaC study. We are grateful for the time and effort they have invested in the project. We thank the patient representatives in the study group, Kristin Isabella Kirkengen Espe and Roger Thoresen. Many people have contributed to the study design and implementation of the study at The Norwegian Institute of Public Health, Akershus University Hospital, Diakonhjemmet hospital, and Oslo University Hospital. We thank all study personnel, laboratory personnel, and other staff at the clinical departments involved.
Disclosure of Interests: Hilde S. Ørbo: None declared, Asia-Sophia Wolf: None declared, Taissa M. Kasahara: None declared, Kristin Hammersbøen Bjørlykke Janssen-Cilag, Ingrid Jyssum: None declared, Joe Sexton: None declared, Anne Therese Tveter: None declared, Guri Solum: None declared, Ingrid Fadum Kjønstad: None declared, Ingrid E. Christensen: None declared, Tore K. Kvien Grünenthal, Janssen, Sandoz, AbbVie, Gilead, Janssen, Novartis, Pfizer, Sandoz, UCB, AbbVie, BMS, Galapagos, Novartis, Pfizer, UCB, Grete B. Kro: None declared, Jørgen Jahnsen AbbVie/Abbott, Bristol-Myers, Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, Takeda, AbbVie/Abbott, Pfizer, Espen A. Haavardsholm Pfizer, UCB, Novartis, Abbvie, Pfizer, Eli Lilly, Ludvig A. Munthe Incyte, Janssen, Sella Aarrestad Provan: None declared, John Torgils Vaage: None declared, Kristin Kaasen Jørgensen Bristol-Myers Squibb, Roche, Gunnveig Grødeland Bayer, Sanofi, ThermoFisher, Pfizer, Vitusapotek, GSK, Siri Mjaaland: None declared, Silje Watterdal Syversen: None declared, Guro Løvik Goll AbbVie/Abbott, Galapagos, Pfizer, UCB.