

Background: At the beginning of the COVID-19 pandemic there were controversial opinions regarding the possibility of temporary interrupting immunomodulatory treatments in rheumatic patients to enhance the immune response to the COVID-19 vaccine. Previous published works have raised the concern that methotrexate (MTX) might have a negative effect on immune response upon COVID-19 vaccine administration in this population. In this context, we postulated that temporary MTX interruption after COVID-19 vaccine administration could be beneficial for rheumatoid arthritis (RA) and psoriatic arthritis (PsA) patients in terms of improving both humoral and cellular responses, based on prior data on influenza vaccine.
Objectives: Primary Objective: To evaluate the impact on B and T cell responses to COVID-19 vaccine of 1 or 2 weeks of MTX withdrawal following each vaccine dose in patients with RA or PsA. Secondary Objective: To analyze the adverse events in terms of flares of the disease.
Methods: Single-centre, randomized, prospective study. Adult RA and PsA patients treated with MTX at stable dose were recruited between March and September 2021 (first vaccination campaign) and randomly assigned to 3 groups: MTX-maintenance, and MTX-withdrawal for 1 wk or 2 wks after each COVID-19 vaccine dose. Samples were collected before and 30 days after complete vaccination. Multi-antigen cytometric bead array assays to detect specific antibodies to several SARS-CoV-2 antigens and ELISPOT assays measuring interferon (IFN)-y and interleukin (IL)-21 were performed. Multivariable analyses with Stata v.14 were used to control the effect of possible confounding variables. The rates of disease relapses were recorded.
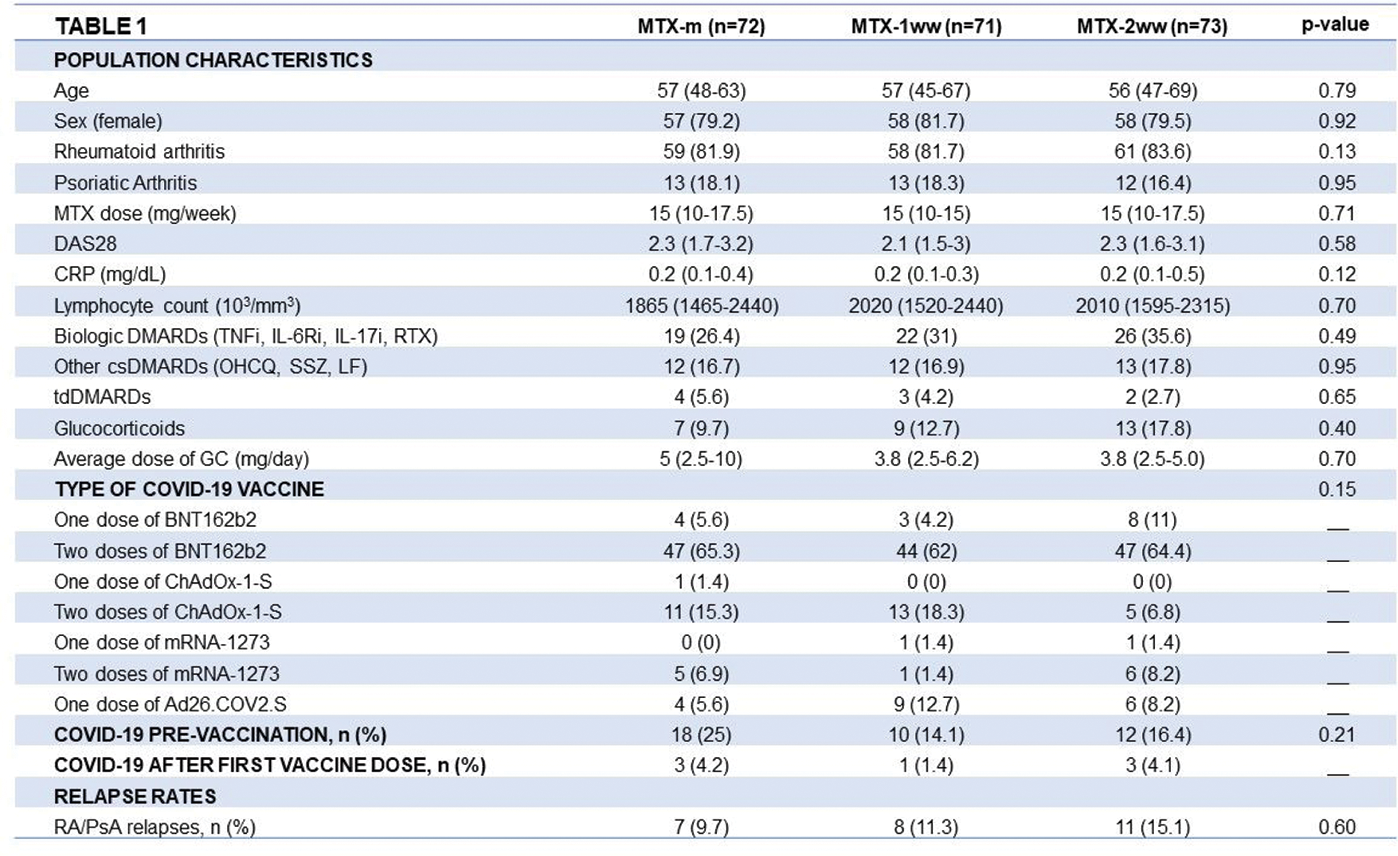
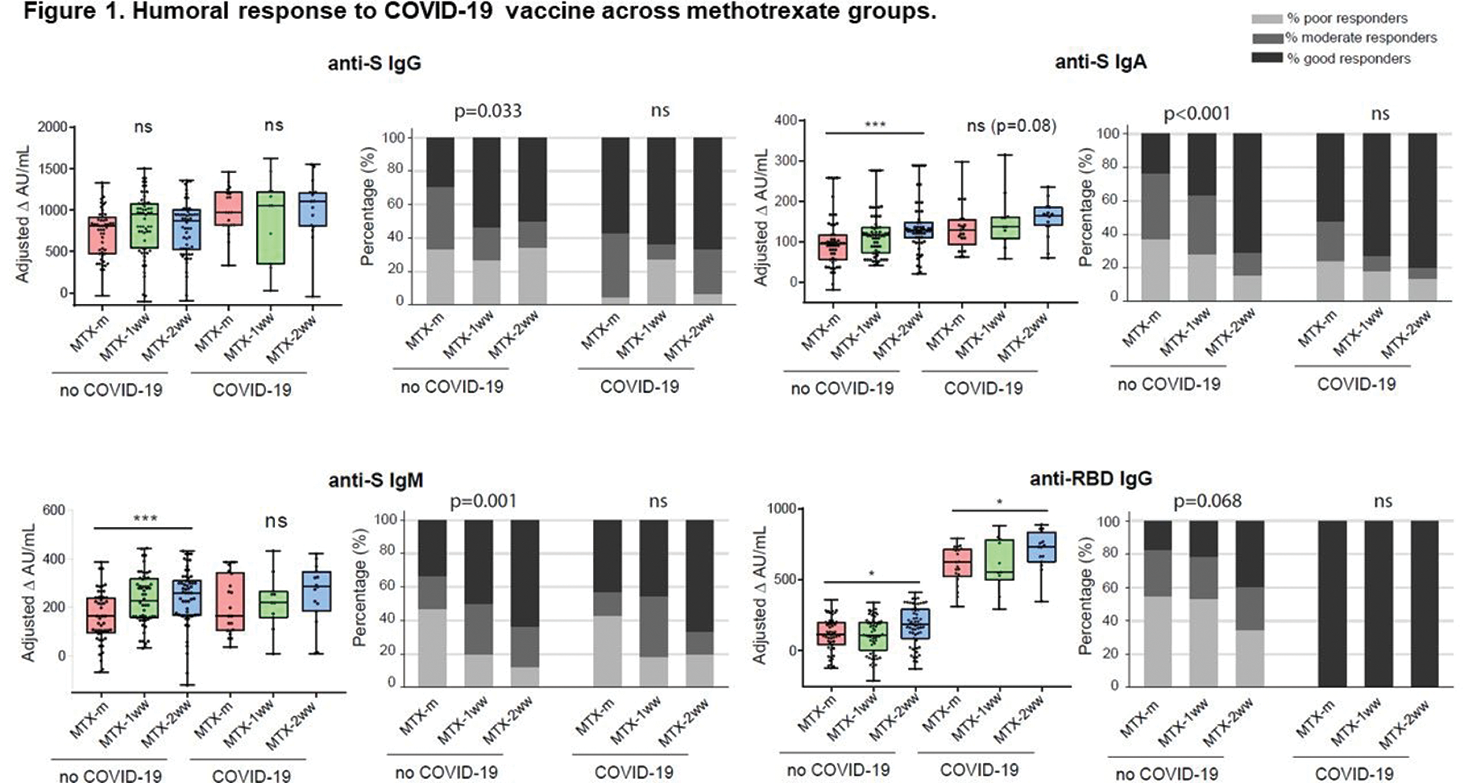
Results: 216 Patients were included (178 RA, 38 PsA), of whom 72 maintained MTX unchanged, and 71 and 73 withdrew MTX for 1 wk and 2 wks, respectively. Population characteristics are shown in Table 1. COVID-19 occurred in 47 patients before complete vaccination. Participants were vaccinated with BNT162b2 (71%), ChadOX-1-S (14%), mRNA-1273 (6%) and Ad26.COV2.S (9%). There were no significant differences in comorbidities (HT, DL, DM, COPD, asthma, CV disease, cancer), types of vaccines or previous COVID-19 infection across groups. On average COVID-19 vaccination elicited good levels of humoral and cellular responses, which were higher in patients previously infected with SARS-CoV-2. Humoral response was higher with mRNA vaccines. MTX withdrawal was associated with a significantly higher number of good responders for anti-S antibodies and significantly higher titres of anti-S IgA and IgM antibodies, anti-RBD IgG antibodies and neutralising antibodies in patients without previous COVID-19, especially in the 2-wks withdrawal group (Figure 1). Regarding cellular response, MTX withdrawal, especially the 2 wks group, was associated with higher IFN-γ secretion both in patients without and with previous COVID-19, but no differences were found for IL-21 across groups. Regarding safety, the rate of relapse was low, either using the DAS criteria (15%) or the physician’s criteria (12%), and the majority were mild or moderate. To highlight, there were no significant differences in the number of RA/PsA relapses across groups. Higher baseline CRP was the only factor associated with relapse: OR 1.77, 95%CI [1.03-3.03], p=0.037.
Conclusion: Our data suggest that a brief MTX interruption following COVID-19 vaccination doses in patients with RA or PsA improves humoral and cellular immune responses, without significant increase of relapses, especially in patients without previous COVID-19 infection and with good disease control.
REFERENCES: NIL.
Acknowledgements: All members of the Rheumatology and Immunology Departments of Hospital Universitario de La Princesa.
Disclosure of Interests: Santos Castañeda: Ministerio de Economía y Competitividad (Instituto de Salud Carlos III) (grant no. PI21/0147 to SC, PID-2020-120412RB-I00 and PDC2021-121797-I00 to FS-M) and co-funded by European regional development fund (ERDF) “A way to make Europe”, and also by REACT-EU-INMUNOVACTER-CM and P2022/BMD7209-INTEGRAMUNE from Comunidad Autónoma de Madrid., Esther Vicente-Rabaneda: None declared, Pedro Martínez-Fleta: None declared, Ana Triguero-Martinez: None declared, Miren Uriarte-Ecenarro: None declared, Francisco Gutiérrez-Rodríguez: None declared, Patricia Quiroga Colina: None declared, Ana Romero: None declared, Noelia García Castañeda: None declared, Jesús A. García-Vadillo: None declared, Cristina Valero: None declared, Irene Llorente: None declared, Ana Ortiz: None declared, Eva Tomero Muriel: None declared, Maria Aranzazu Alfranca: None declared, Rosario Garcia-Vicuña: None declared, Francisco Sánchez-Madrid: None declared, Isidoro González-Álvaro: None declared.