

Background: Pseudogout is an acute arthritis induced by calcium pyrophosphate dehydrate crystal deposition (CPPD) [1]. Pseudogout most commonly involves one or several large joints, such as the knee or wrist, typically tender and swollen [2]. The pathogenic mechanism of CPPD is partially understood, and the etiology of attacks is unclear. There is no diagnostic serum biomarker for pseudogout. CPPD disease is best diagnosed by identifying positively birefringent, typically rhomboid-shaped crystals in the synovial fluid of affected joints. However, CPPD disease and rheumatoid arthritis (RA) often coexist, and the similarity of pseudogout with acute flare of RA might lead to misdiagnosis.
Objectives: To identify the serum differential diagnostic biomarkers for pseudogout and RA using the results of metabolomic analysis.
Methods: We collected the serum samples of 18 pseudogout and 12 RA patients who showed acute arthritis. We also collected the serum of five pseudogout patients after improving their arthritis. We performed metabolomic analysis of the samples using a gas chromatography mass spectrometer (GC-MS). For detecting the metabolites which characterizing pseudogout with arthritis, the metabolic profiles were compared using the orthogonal partial least-squares discriminant analysis (OPLS-DA) weightings (variable importance in the projection (VIP) > 1, the absolute value of modeled correlation (|p(corr)|) > 0.5 in the S-plot) and Mann-Whitney U test ( p < 0.05). Next, for assessment the utility of the detecting metabolites as novel biomarkers, we performed validation study using additional serum samples of 11 pseudogout and 13 RA patients.
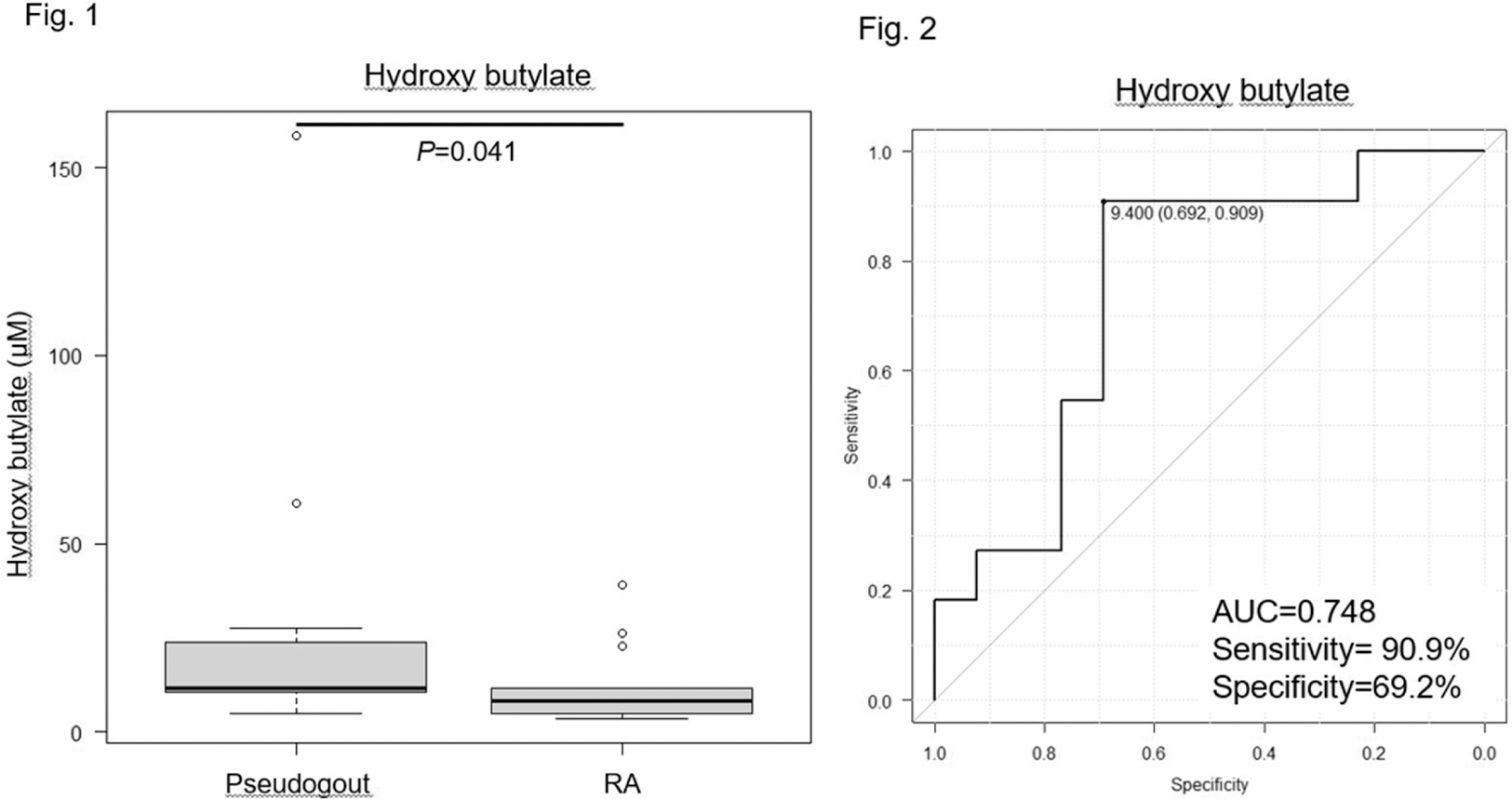
Results: A total of 101 metabolites were identified by GC-MS. By using univariate and multivariate statistical analysis, 19 metabolites were different between pseudogout and RA patients. Nine metabolites were different between pseudogout patients showing arthritis and after improving their arthritis. As results from analysis, we identified hydroxy butyrate and 2,3-bisphospho-glycerate, which elevating in pseudogout patients with arthritis. As the result from validation study, the levels of hydroxy butyrate in pseudogout patients was significantly higher than RA patients (p = 0.041) (Figure 1). The receiver operating characteristic(ROC)analysis for hydroxy butyrate as biomarker revealed an area under the curve (AUC) of 0.748, with a sensitivity of 90.9% and specificity of 69.2% at the cutoff level of 9.40 μM (Figure 2).
Conclusion: We identified hydroxy butyrate as the potential differential diagnostic serum biomarker for pseudogout and RA.
REFERENCES: [1] McCarty DJ et al. Ann Intern Med 1962;56:711-737.
[2] McCarthy GM and Dunne A. Nat Rev Rheumatol 2018 Oct;14(10):592-602.
Acknowledgements: NIL.
Disclosure of Interests: None declared.