

Background: Gout, the most common form of inflammatory arthritis (affecting 5.2% of US adults), is a metabolic condition influenced by genetics as well as environmental factors. Small-scale, cross-sectional studies of Asian men with preexisting gout implicated certain amino acids, but their generalizability beyond Asian countries or in women is unclear.
Objectives: To prospectively investigate the pre-diagnostic, population-based metabolome for risk of hospitalized gout (i.e., accurate, severe, and costly cases; PPV 100% for gout[1]), accounting for baseline serum urate levels.
Methods: We conducted pre-diagnostic metabolome-wide analysis among 249,677 UK Biobank participants (45% male, mean age 57.2 years at baseline) with NMR metabolomic profiling (N=168 metabolites, including eight amino acids) from baseline blood samples (2006-2010), without history of gout. We calculated multivariable hazard ratios (HRs) for incident hospitalized gout, before and after adjusting for serum urate levels; we included non-hospitalized incident gout cases in a sensitivity analysis. Potential causal effects were evaluated with two-sample Mendelian randomization using genetic association data from the GlobalGout consortium (N=100,661 cases and 2,106,003 controls).
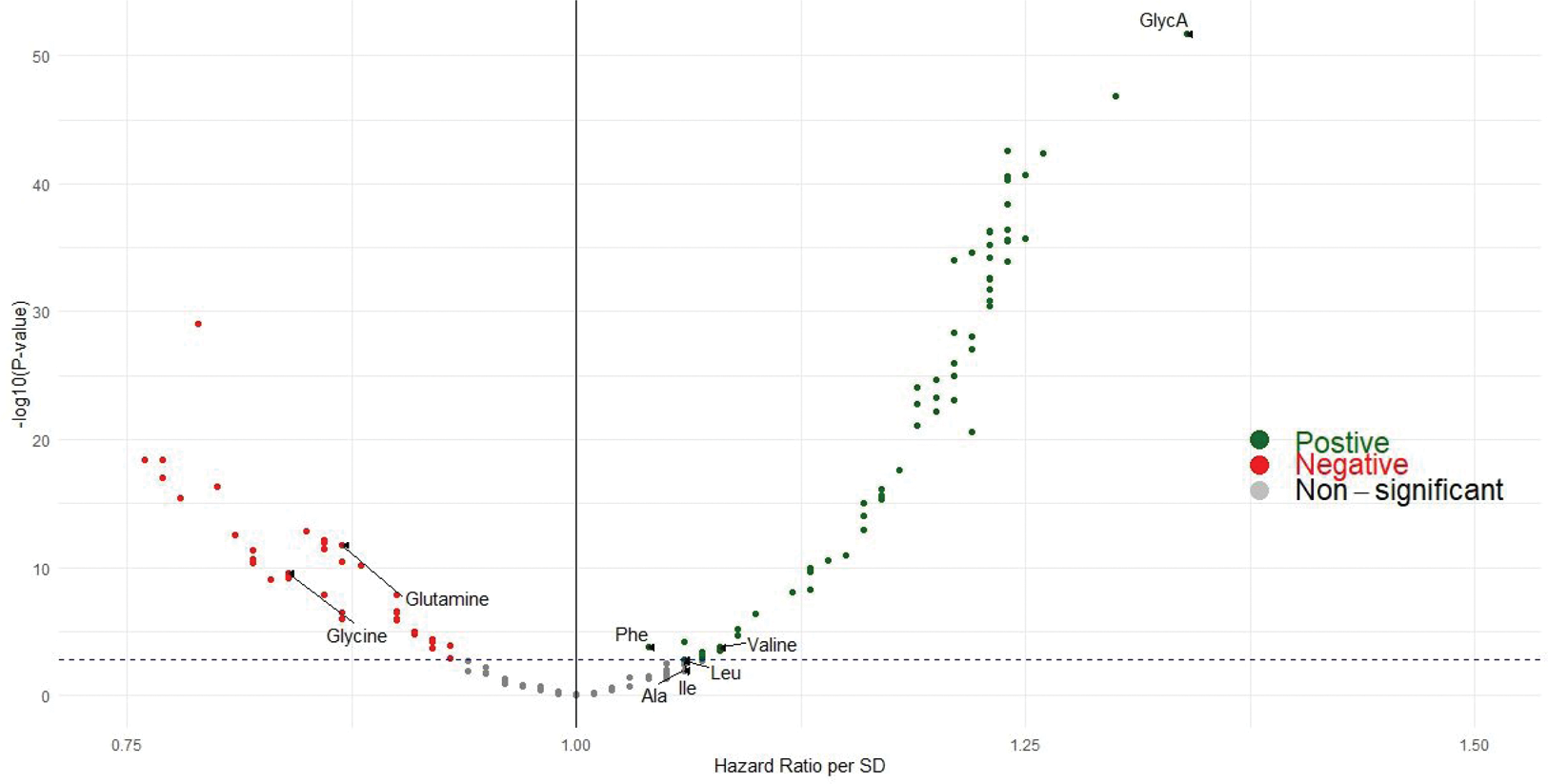
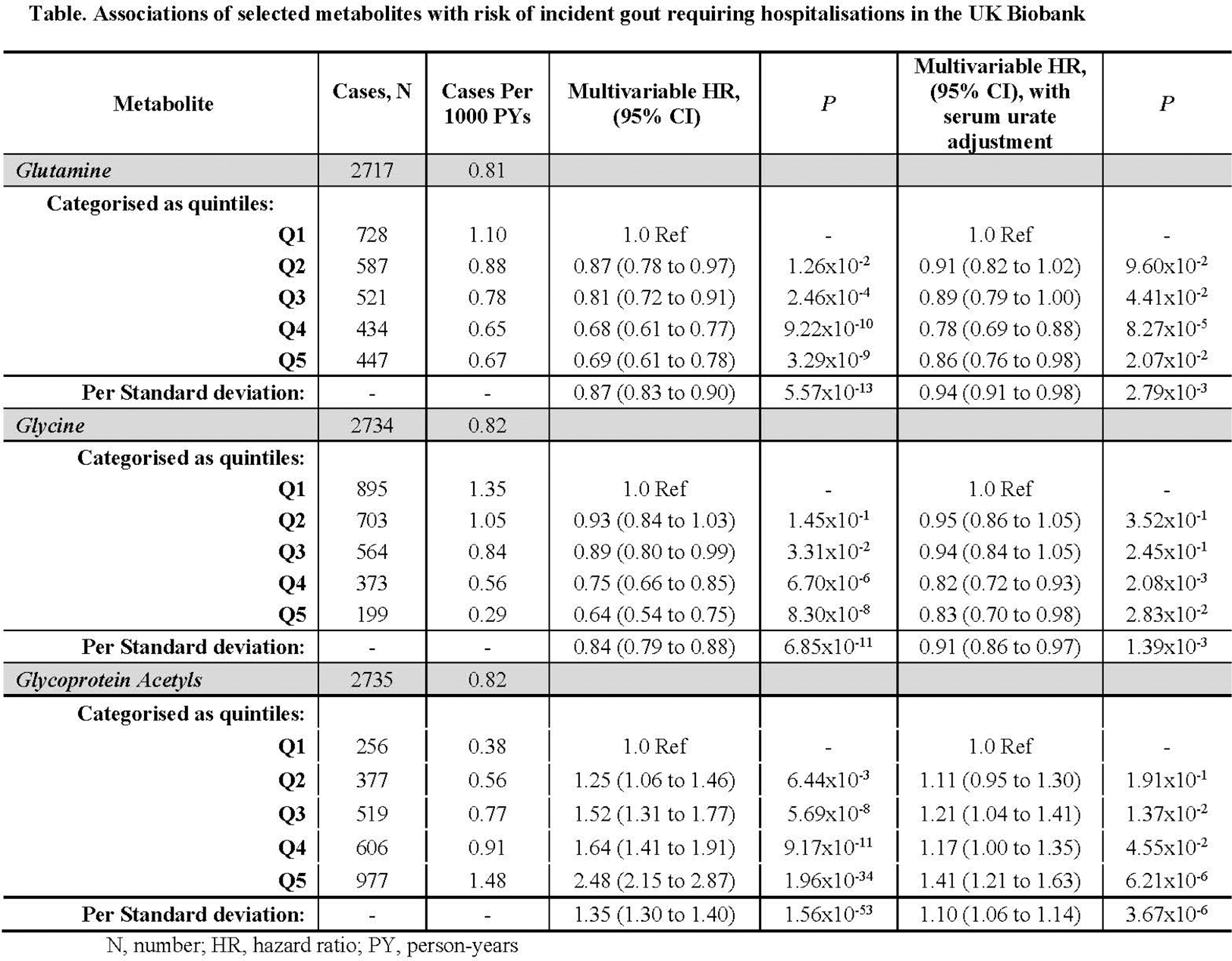
Results: Correcting for multiple testing, over a mean follow-up of 13.4 years, 107 metabolites were associated with incidence of hospitalized gout (N=2735 cases) before urate adjustment (Figure 1), including glycine and glutamine (inversely; HR=0.64 [95% CI: 0.54, 0.75], P=8.3x10 -8 and HR=0.69 [0.61, 0.78], P=3.3x10 -9 between extreme quintiles, respectively), the branched chain amino acids isoleucine, leucine, and valine (inversely), and the inflammatory biomarker glycoprotein acetyls (GlycA; HR=2.48 [2.15, 2.87], P=1.96x10 -34 ) (Table 1). The associations for glycine, glutamine, and GlycA remained significant and directionally-consistent following urate adjustment (HR=0.83 [95% CI: 0.70, 0.98], 0.86 [0.76, 0.98], 1.41 [1.21, 1.63] between extreme quintiles), respectively; corresponding HR per SD were 0.91 (0.86, 0.97), 0.94 (0.91, 0.98), and 1.10 (1.06, 1.14). Findings persisted when including non-hospitalized incident gout cases. Mendelian randomization corroborated the potential causal role of these amino acids on hyperuricemia or gout risk; with change in urate levels of -0.05 mg/dL (-0.08, -0.01), and -0.12 mg/dL (-0.22, -0.03), per SD of glycine and glutamine, respectively, and ORs 0.94 (0.88, 1.00), and 0.81 (0.67, 0.97), for gout. MR-Egger intercepts suggested no directional pleiotropy.
Conclusion: These findings are consistent with glutamine and glycine’s documented immune modulatory properties, including inhibition of activation of the NLRP3 inflammasome, a key component in the pathogenesis of gout flares, and experimental evidence suggesting glutamine levels are lower in synovial fluid samples taken during gout flares. From a clinical standpoint, these prospective findings with causal implications suggest a potential role for glycine and glutamine supplementation in lowering gout risk, as supported by trials where glycine or glutamine supplementation decreased insulin resistance[2] and the inflammatory response,[3] and increased urinary uric acid excretion.[4]
REFERENCES: [1] Singh. Arthritis Res Ther 2013.
[2] Alves et al. Nutrients 2019.
[3] Ortiz de Urbina et al. Int J Med Sci 2017.
[4] Oshima et al. Nutrients 2019.
Volcano plot for metabolome-wide associations for incident gout.
The metabolites associated in our hypothesis-driven, targeted analysis (α=0.05) are also marked with labels; glycine and glutamine were inversely associated with gout risk. Ala, alanine; Ile, isoleucine; GlycA, glycoprotein acetyls; Leu, leucine; Phe, phenylalaine; Val, valine.
Acknowledgements: NIL.
Disclosure of Interests: Natalie McCormick: None declared, Amit Joshi Regeneron Pharmaceuticals, Regeneron Pharmaceuticals, Chio Yokose: None declared, Bing Yu: None declared, Adrienne Tin: None declared, Robert Terkeltaub Recently served or currently serves as a consultant for Allena, LG Chem, Fortress/Urica, Selecta Biosciences, Horizon Therapeutics, Atom Bioscience, Acquist Therapeutics, Generate, Biomedicines, Astra-Zeneca, and Synlogic., Astra-Zeneca (previous), Tony Merriman: None declared, Oana Zeleznik: None declared, A. Heather Eliassen: None declared, Gary Curhan Chief Medical Officer at OM1, Inc, GSK, Hang Korng Ea: None declared, Matthew Nayor: None declared, Laura Raffield: None declared, Hyon Choi Ani, LG, Horizon, Shanton, Protalix, Horizon.