

Background: Carotid atherosclerosis is prevalent in patients with gout. We previously identified an association between tophi and positive Power Doppler (PD) signal with atheroma plaques in a cross-sectional study [1]. However, confirmation through a longitudinal approach is needed.
Objectives: To explore the association between the evolution of carotid atherosclerosis with changes in sonographic crystal deposits and associated inflammation.
Methods: We report the prospective follow-up of our inception cohort, which included 103 patients with recently diagnosed crystal-proven gout, 94% men, with a mean baseline age of 62 years, and 17% with tophaceous disease. Three ultrasound assessments were performed: baseline (M0), at six months (M6), and at 12 months (M12). The same sonographer, blinded to clinical data, performed the repeated assessments. M0 included A) scans of bilateral carotid to evaluate intima-media thickness (IMT) and atheroma plaques presence, according to Mannheim consensus, and B) musculoskeletal scans in wrists, second metacarpophalangeal and first metatarsophalangeal joints, and tricipital and patellar tendons, evaluating crystal deposit signs (double contour, aggregates, and tophi, according to OMERACT definitions) and local PD signal, graded from 0 to 3. M0 was performed without urate-lowering therapy, but prophylactic agents were allowed. Subsequently, patients were treated in parallel by their rheumatologists in clinical grounds, who were aware of the scan results. At M6 and M12, carotid and musculoskeletal ultrasounds were repeated at locations with baseline deposits. The sonographer classified the evolution of deposits and carotid plaques as “disappearance,” “reduction,” “persistence,” and “progression”. We later dichotomized plaque and deposit variables into “disappearance/reduction” or “persistence/progression” for statistical analyses. The maximum grade in all evaluated locations was taken for the PD signal. The association between changes in carotids, deposits, and maximum PD signal was studied using the Chi-square test and Fisher’s exact test. IMT changes using the t-test for repeated measures.
Results: Of 103 patients, 91 (88.3%) and 78 (75.7%) underwent M6 and M12 evaluations, respectively. At M0, the mean number of locations with deposits and a positive PD signal was 9.9 (SD 4.1) and 1.1 (SD 1.1). Fifty-nine percent had carotid atheroma plaques; the mean IMT was 0.82 mm (SD 0.21). Figure 1 shows the evolution of carotid plaques, urate deposits, and PD signal (Figure 1A M0-M6; Figure 1B M6-M12). IMT persisted unchanged at M6 (p=0.801) and M12 (p=0.822). At M6, no association was found between carotid and musculoskeletal variables. However, at M12, the reduction or disappearance of atheroma plaques was significantly associated with the absence of a PD signal, compared to a persistent PD signal (64.0% vs. 28.6%, p=0.017). Improvement in carotid plaques was unrelated to improvements in double contour signs (62.5% vs. 0%, p=0.087), aggregates (57.9% vs. 37%, p=0.162), or tophi (48.4% vs. 42.9%, p=0.731). High-intensity statins was also associated with the reduction or disappearance of atheroma plaques. No relationship was found with other clinical, laboratory or treatment variables.
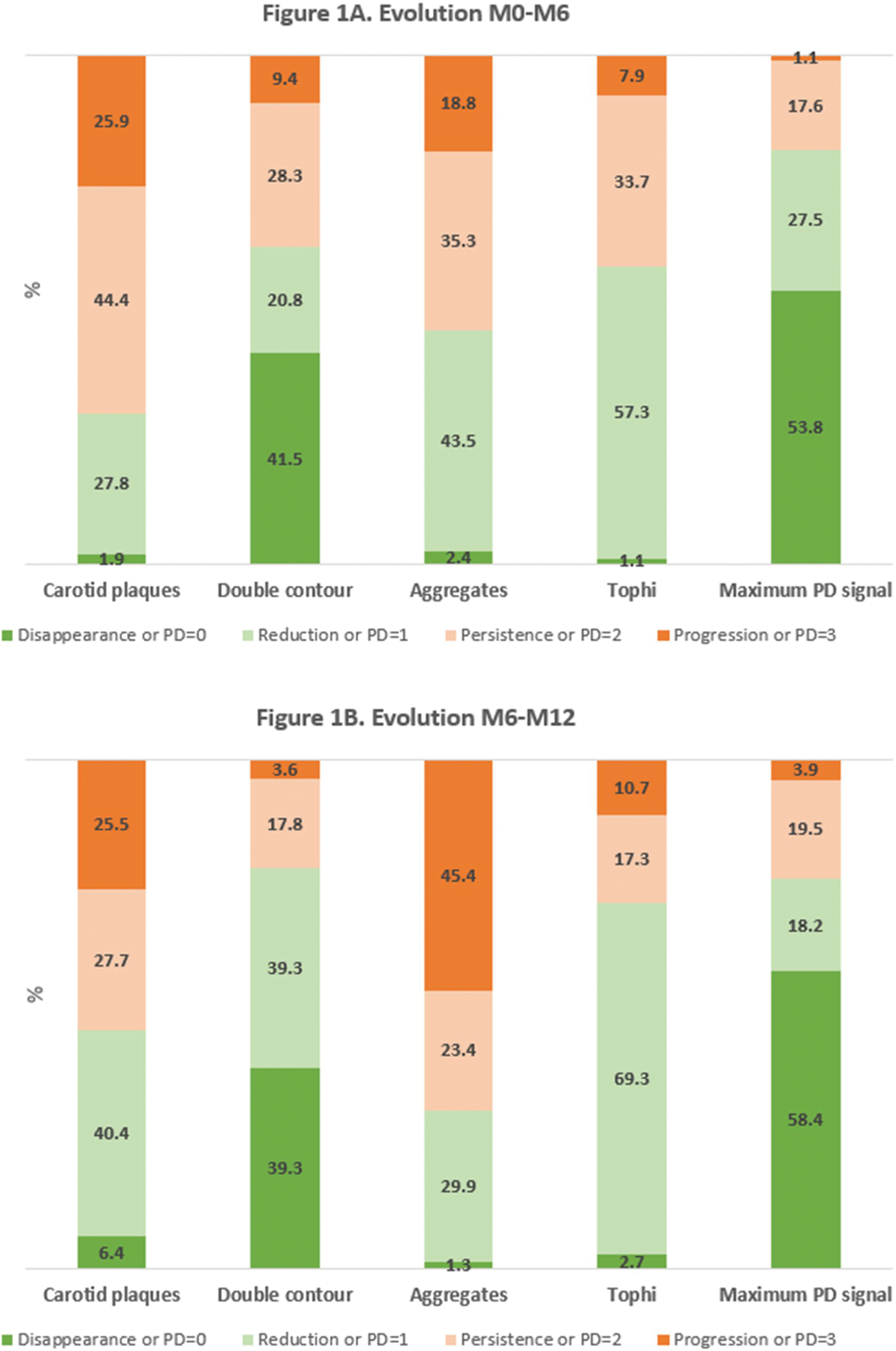
Conclusion: After 12 months of urate-lowering treatment in gout patients, improvements in carotid atheroma plaques were more common in those with an absent PD signal. This supports the relationship identified at baseline and adds evidence to the link between crystal-driven inflammation and atherosclerosis in patients with gout.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Mariano Andrés Menarini, Grunenthal, Cristina Rodríguez: None declared, Irene Calabuig: None declared, Agustín Martínez-Sanchis: None declared.