

Background: Systemic lupus erythematosus (SLE) is characterized by a wide diversity of clinical manifestations, and the etiology may vary among patients. Immunophenotyping of peripheral blood has revealed dysregulation in the differentiation of B cells compared to that in healthy individuals. However, the correlation between these differences and clinical symptoms remains unclear. Moreover, the appropriate sub-grouping of lupus patients holds the potential to pave the way for precision medicine.
Objectives: The purpose of this study was to identify peripheral blood immunological abnormalities in SLE, assess their diversity, and attempt to subgroup lupus patients. Additionally, we investigated the relationship between these subgroups and clinical symptoms as well as prognosis.
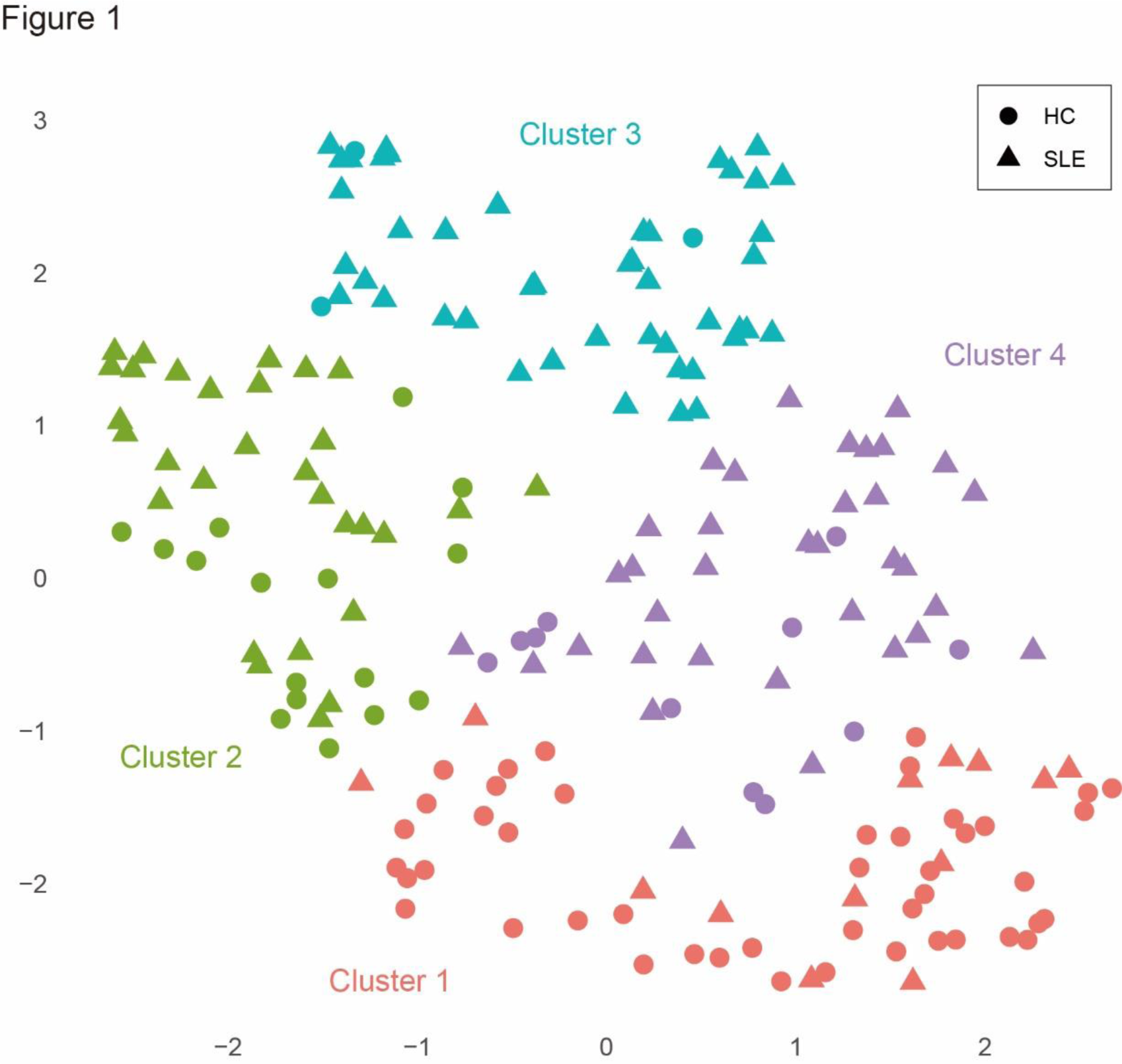
Methods: Immunophenotyping of immunocompetent cells in peripheral blood, encompassing T cells, B cells, NK cells, dendritic cells, and monocytes, was conducted using multicolor flow cytometry following the antibody set proposed by NIH/FOCIS. The analysis included 123 untreated patients with SLE and 75 healthy subjects. The immunophenotypes were assessed as a percentage within peripheral blood mononuclear cells. The outcomes underwent cluster analysis, employing both the Ward’s method with Euclidean distance to stratify the patient population. Based on the tree diagram, the number of clusters was set to four. Visualization of the clusters was performed using Uniform Manifold Approximation and Projection (UMAP) method. Clinical symptoms and recurrence rates at 3 years were evaluated within each subgroup.
Results: All patients in the study were untreated, with a median age of 42 years. The median disease duration was 5 months. The disease activity, as measured by SLEDAI, averaged 11, and 66% of the patients had either one BILAG A or two Bs (BILAG A1 or B2). Overall, lupus patients exhibited an elevation in double-negative B cells (1.4% vs 0.6%) and plasmablasts (1.4% vs 0.2%) in peripheral blood compared to healthy controls. In T cells, there was an elevation in activated CD4 (1.8% vs 0.6%), activated CD8 (4.7% vs 0.6%), and activated Th1 cells (0.8% vs 0.3%). Additionally, there was a reduction in CD16 + NK cells (5.6% vs 10.3%). Cluster analysis based on this immunophenotype stratified untreated SLE patients into four major groups and visualized them using UMAP (Figure 1). One of the four clusters exhibited an almost identical immunophenotype to that of healthy controls and comprised the smallest number of patients (cluster 1). The remaining three clusters were nearly evenly distributed, distinguished by a relatively low proportion of plasmablasts with a notable increase in double-negative B cells (cluster 2), a tenfold increase in plasmablasts (cluster 3), and an elevation in activated regulatory T cells (cluster 4). Cluster 2 was the youngest in terms of age, with no differences observed in disease duration or gender. In these four clusters, cluster 1 exhibited the lowest disease activity, with BILAG A1 or B2 at 40%, whereas cluster 3, characterized by markedly elevated plasmablasts, showed high disease activity, with BILAG A1 or B2 at 88%, and hypocomplementemia was also prevalent. An analysis of the 3-year clinical course of patients undergoing high-dose glucocorticoid therapy revealed that cluster 1 did not experience a single relapse, while cluster 3 had more than 20% relapses. The remaining clusters exhibited approximately 10% relapses.
Conclusion: Immunophenotyping facilitated the stratification of untreated lupus patients into four distinct groups. While SLE is characterized by an elevation of double-negative B cells and plasmablasts, these immune cell subsets exhibited differential behavior within the SLE population. Cluster characterized by a significant increase in plasmablasts exhibited notably high disease activity and relapse rates.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Satoshi Kubo has received speaking fees from Eli Lilly, GlaxoSmithKline, Bristol-Myers, Abbvie, Eisai, Pfizer, Astra-Zeneca, has received research grants from Daiichi-Sankyo, Abbvie, Boehringer Ingelheim, and Astellas., Shingo Nakayamada has received speaking fees from Bristol-Myers, UCB, Astellas, Abbvie, Eisai, Pfizer, and Takeda, has received research grants from Mitsubishi-Tanabe, Novartis, and MSD, Yusuke Miyazaki has received epeaking fees from Astra-Zeneca, speaking fees, and/or honoraria from Eli Lilly and GlaxoSmithKline, Yasuyuki Todoroki: None declared, Masanobu Ueno: None declared, Katsuhide Kusaka: None declared, Satsuki Matsunaga: None declared, Hiroaki Tanaka: None declared, Naoaki Ohkubo: None declared, Hidenori Sakai: None declared, Ippei Miyagawa: None declared, Kentaro Hanami: None declared, Yoshiya Tanaka has received speaking fees and/or honoraria from Eli Lilly, AstraZeneca, Abbvie, Gilead, Chugai, Behringer-Ingelheim, GlaxoSmithKline, Eisai, Taisho, Bristol-Myers, Pfizer, Taiho, has received research grants from Mitsubishi-Tanabe, Eisai, Chugai, Taisho.