

Background: Extracellular vesicles (EVs) deliver small molecules across several cell types including microRNA (miRNA) which are involved in regulation of gene expression and were proven to play a role in several autoimmune rheumatic diseases. Idiopathic inflammatory myopathies (IIM) display heterogeneity in both phenotype and pathogenic mechanisms which may inform on disease course and prognosis.
Objectives: To investigate the epigenetic footprint in IIM through characterization of circulating EVs and the expression of EV-derived small non-coding RNAs (sncRNAs).
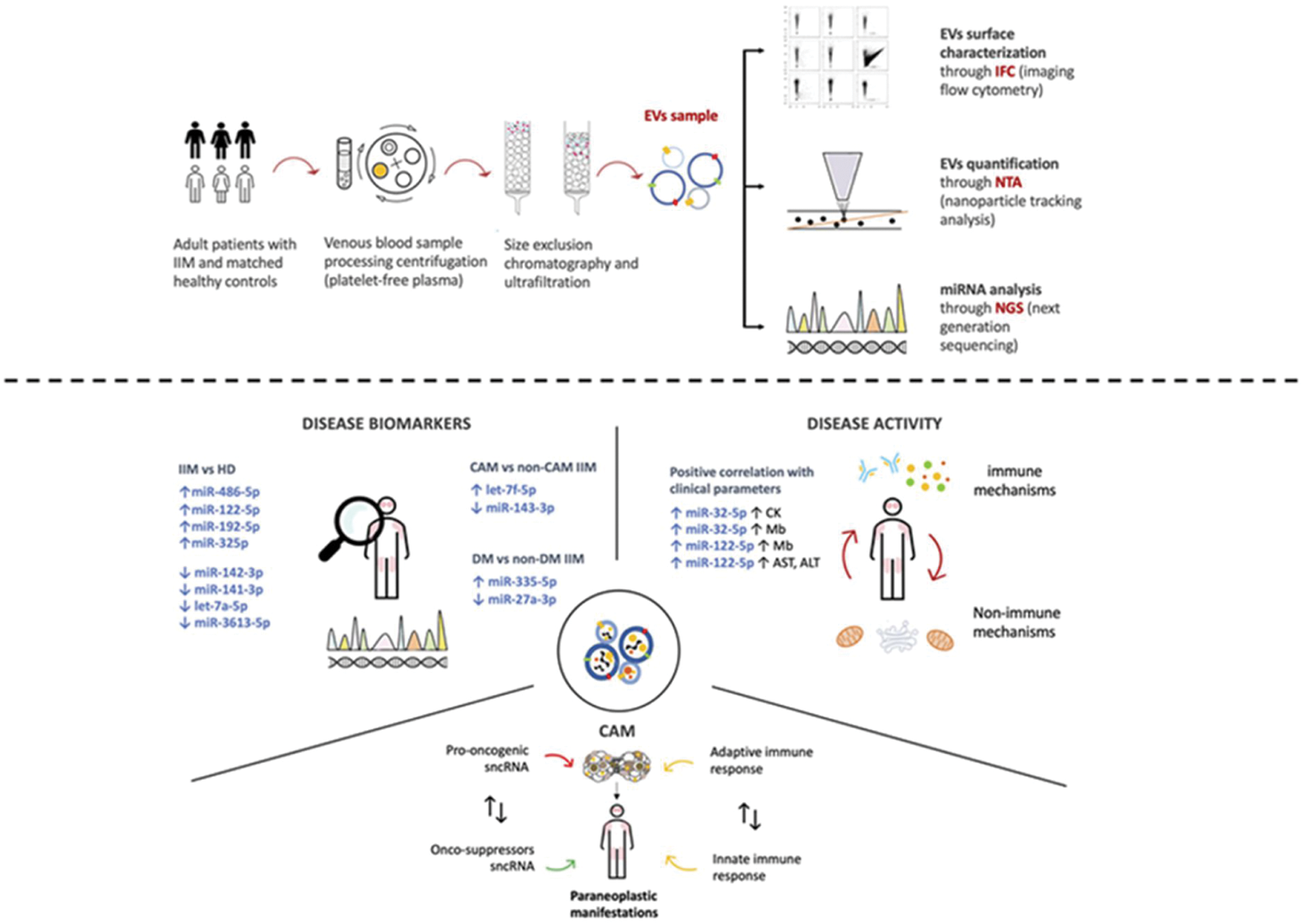
Methods: In this cross-sectional study, EVs were isolated by size-exclusion chromatography from plasma of patients with IIM and matched healthy donors (HD). EV-derived sncRNAs were sequenced and quantified using Next-Generation Sequencing (NGS). Following quality control and normalization, filtered count reads were used for differential miRNAs expression analyses. Putative gene targets enriched for pathways implicated in IIM were analyzed. Patients’ clinical and laboratory characteristics at the time of sampling were recorded.
Results: Forty-seven IIM patients and 45 HD were enrolled. We showed a significant upregulation of MiR-486-5p (p<0.01), miR-122-5p, miR-192-5p, and miR-32-5p (p<0.05 for all), while a downregulaion of miR-142-3p (p<0.001), miR-141-3p (p<0.01), let-7a-5p (p<0.05) and miR-3613-5p (p<0.05) in EVs from IIM patients versus HD (Figure 1). MiR-486-5p was associated with increased muscle enzymes levels. Several target genes of up/downregulated miRNAs in IIM participate in inflammation, necroptosis, interferon and immune signaling. Within IIM, miR-335-5p was selectively upregulated and miR-27a-5p downregulated in dermatomyositis (n=21, p<0.01). Plasma EV levels were significantly increased in cancer-associated myositis (CAM, n=12) versus non-CAM IIM (n=35, p=0.02) and HD (p<0.01). EVs cargo in CAM was significantly enriched of let-7f-5p and depleted of miR-143-3p.
Conclusion: Through an unbiased screening of EV-derived sncRNAs, we show differential patterns of miRNAs expression in the EVs cargo of different IIM subtypes, submitting their role as potential biomarkers and modifiers of diverse IIM phenotypes.
REFERENCES: NIL.
Study design and main results
Acknowledgements: NIL.
Disclosure of Interests: Mariele Gatto GSK, AZ, Janssen, Chiara Franco: None declared, Alessandra Giannella: None declared, Michela Gasparotto: None declared, Anna Ghirardello: None declared, Elisabetta Zanatta Janssen, Luca Iaccarino GSK, AZ, GSK, Giulio Ceolotto: None declared, Andrea Doria GSK, AZ, Pfizer, Celgene, Eli Lilly, BMS, Roche, GSK.