

Background: Glucocorticoids (GCs) are effective in the initial control of active granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), two types of ANCA-associated vasculitis (AAV). However, GCs are associated with a range of well-described toxicities including: increased risk of infection, diabetes mellitus, weight gain, elevated blood pressure and sleep and neuropsychiatric disturbances. Patients report detrimental effects on health-related quality of life (HRQoL) from GC use. In the phase 3 ADVOCATE trial of avacopan vs prednisone taper, non-study supplied GCs were allowed and used by patients in both arms if they had worsening disease. However, 28 patients in the avacopan arm did not receive GCs during the first 29 days of the trial, when GCs are typically given at high doses. Eleven patients did not receive any GCs at any time, either in pre-screening, screening, or during the trial.
Objectives: The objective was to describe the baseline characteristics and outcomes of patients who received avacopan but no GCs (N=28, 17% of 166 patients who received avacopan in ADVOCATE) during the first 29 days after screening and randomization in the trial, including 11 patients with no GC use at any point.
Methods: Outcomes for this analysis of an intent-to-treat subgroup were remission at week 26 and sustained remission at week 52. Other outcomes included relapse rate, change in estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR), GC dose (prednisone-equivalent), HRQoL, and safety.
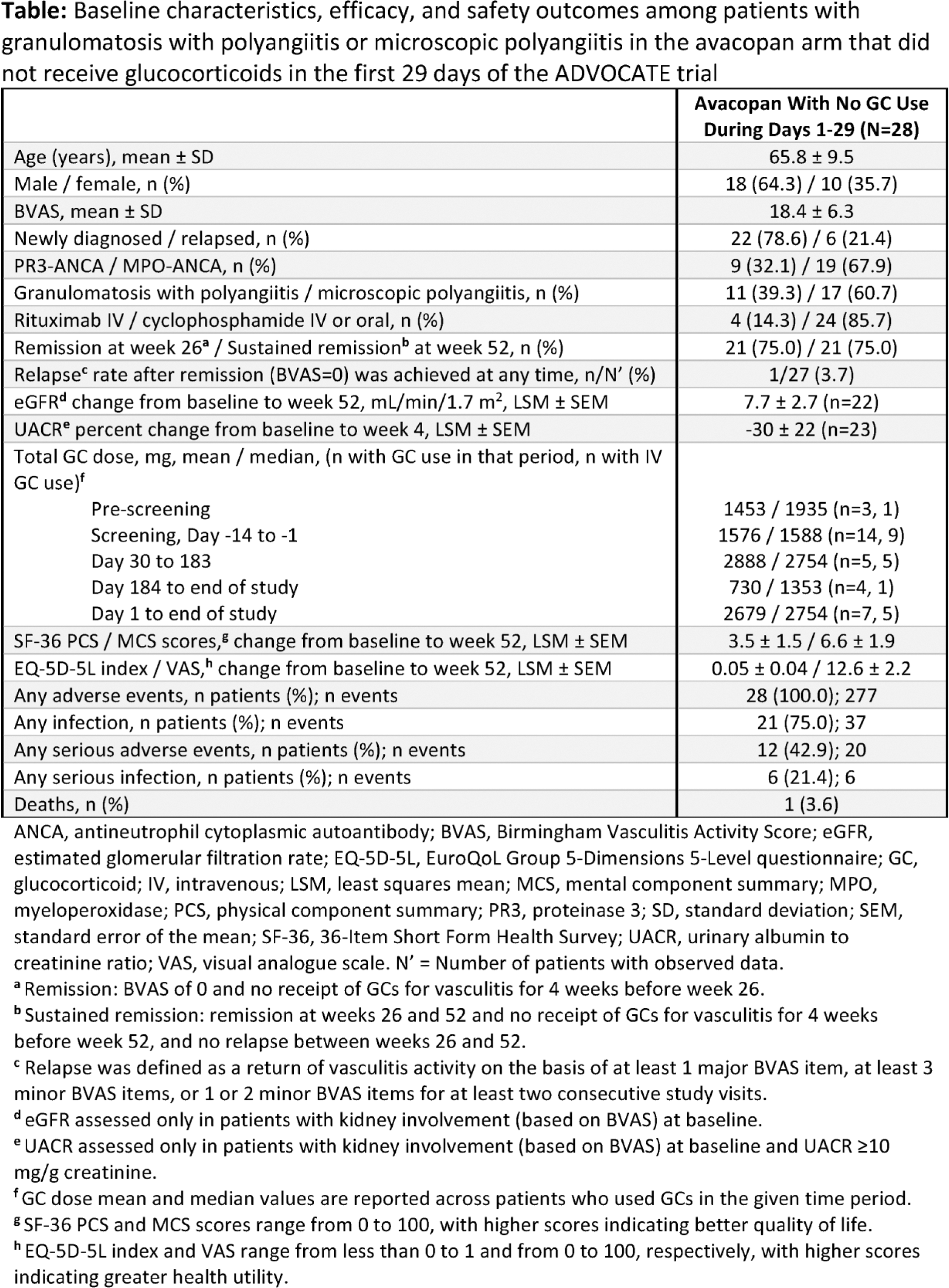
Results: Baseline characteristics are listed in the Table 1 . Of the 28 patients, 22 (78.6%) were newly diagnosed, 17 (60.7%) had MPA, 26 (92.9%) had active kidney manifestations at baseline, and 4 (14.3%) received rituximab (RTX) as background therapy. Fourteen (50.0%) patients used GCs in screening, with 9 (32.1%) receiving intravenous GCs. Birmingham Vasculitis Activity Score (BVAS) was 18.4 ± 6.3 (mean±SD) at baseline, compared with 16.3 ± 5.9 for the overall ADVOCATE trial population. The same proportion of patients (75.0%) who achieved remission at week 26 also had sustained remission at week 52, indicating no patients relapsed after week 26. The GC dose (mean/ median) across those that used GCs during a given time period is listed in the Table 1 and was 1576/ 1588 mg during screening and 2679/ 2754 mg during days 1 to end of study. Six patients received GCs after day 29, 4 of which did not achieve remission; two did achieve and sustain remission and received GCs to treat non-AAV symptoms. Kidney function outcomes improved, with a least squares mean (LSM) increase in eGFR of 7.7 mL/min/1.73 m 2 at week 52 and an LSM change of -30% in UACR at week 4. All measures of HRQoL improved at week 52 ( Table 1 ). Infections were the most common adverse event (AE, 75.0%); one death occurred (3.6%) due to pneumonia on day 160. One patient stopped treatment at day 7 (positive tuberculosis test). Of 11 patients who did not receive GCs at any point, 9 (81.8%) were male, 7 (63.6%) were newly diagnosed, 8 (72.7%) had MPA, 8 (72.7%) had MPO-ANCA, 10 (91.0%) had kidney manifestations and 2 (18.2%) received RTX, with a mean age of 63.3 ± 9.6 years and BVAS of 17.3 ± 5.9 at baseline. All 11 patients (100%) achieved remission at week 26 and sustained remission at week 52; none relapsed. Mean eGFR change was 8.4 mL/min/1.73 m 2 at week 52 and mean UACR change was -13% at week 4. Infection was the most common AE (64%, 12 events) followed by rash (45%, 7 events); serious AEs were reported in 5 patients.
Conclusion: Findings from this post hoc analysis of ADVOCATE involving 28 patients with GPA/MPA who did not receive concomitant GCs during the first 29 days of the trial demonstrated efficacy and safety of avacopan. For a smaller group of 11 patients, GCs were not used throughout the trial, and all patients achieved remission at week 26 and sustained remission at week 52, indicating this may be a potential treatment course option in this population type. GC use should be individualized to achieve the best outcomes for patients with GPA/MPA.
REFERENCES: NIL.
Acknowledgements: The authors would like to thank the patients, personnel, and investigators at ADVOCATE sites throughout the world. We also thank Cactus Life Sciences (part of Cactus Communications), Mumbai, India, for their editorial support.
Disclosure of Interests: John H. Stone ChemoCentryx (Instructor); Amgen (Instructor), ChemoCentryx (Consultant); Amgen (Consultant), David R. W. Jayne Amgen (Speakers bureau); GSK (Speakers bureau); CSL Vifor (Speakers bureau), Alentis (Shareholder); Aurinia (Shareholder), AstraZeneca (Consultant); Boehringer Ingelheim (Consultant); Chinook (Consultant); CSL Vifor (Consultant); GSK (Consultant); Novartis (Consultant); Roche (Consultant), GSK (Grant/research support); Roche (Grant/research support); CSL Vifor(Grant/research support), Sarah Bray Amgen (Employee), Darcy Trimpe Amgen (Employee), Rachel E. Gurlin Amgen (Employee), Peter A. Merkel Kyverna (Stock options), Amgen (Consultant); Argenx (Consultant); AstraZeneca (Consultant); Boehringer Ingelheim (Consultant); Bristol Myers Squibb (Consultant); Cabaletta (Consultant); CSL Behring (Consultant); GSK (Consultant); HiBio (Consultant); InflaRx (Consultant); Janssen (Consultant); Jubilant (Consultant); Kyverna (Consultant) MiroBio (Consultant); Novartis (Consultant); NS Pharma (Consultant); Q32 (Consultant); Regeneron (Consultant); Sanofi (Consultant); Sparrow (Consultant); Takeda (Consultant); UpToDate (Royalties); Visterra (Consultant), AbbVie/Abbott (Grant/research support); Amgen (Grant/research support); AstraZeneca (Grant/research support); Boehringer Ingelheim (Grant/research support); Bristol Myers Squibb (Grant/research support); Eicos (Grant/research support); Electra (Grant/research support); Genentech (Grant/research support); GSK (Grant/research support); InflaRx (Grant/research support); Neutrolis (Grant/research support); Takeda (Grant/research support).