

Background: Immunoglobulin-A vasculitis (IgAV) is an inflammatory vascular disease [1, 2]. The defining pathophysiologic feature of this vasculitis is the elevated levels of an aberrantly glycosylated galactose-deficient IgA (gd-IgA1) in circulation [3]. These elevated gd-IgA1 levels lead to glycan-specific IgG antibody development [4], which forms circulating IgA1-IgG anti-IgA1 immune complexes that ultimately deposit in different tissues, causing inflammation [1]. Consequently, B-cells are proposed as crucial players in the pathogenesis of IgAV. Nevertheless, the molecular mechanisms by which these B-cells are involved in IgAV are still unknown.
Objectives: To identify the molecular mechanisms by which B-cells are implicated in the pathogenesis of IgAV, by performing the first exhaustive analysis of the methylome of peripheral B-cells.
Methods: 30 Caucasian patients diagnosed with IgAV, who were in the acute phase of the disease and had not received previous treatment to control of this vasculitis, and 30 ancestry-age and sex-ethnically matched healthy controls were recruited in this study. Peripheral blood mononuclear cells were isolated from all the IgAV patients and healthy controls by Ficol and density gradient. Then, B-cells were purified using magnetic cell separation by MACS® Technology. Finally, genomic DNA was extracted from each B-cell and, subsequently, was bisulfite-converted with the EZ DNA Methylation TM kit, and hybridized onto an Infinium MethylationEPIC Bead Chip array.
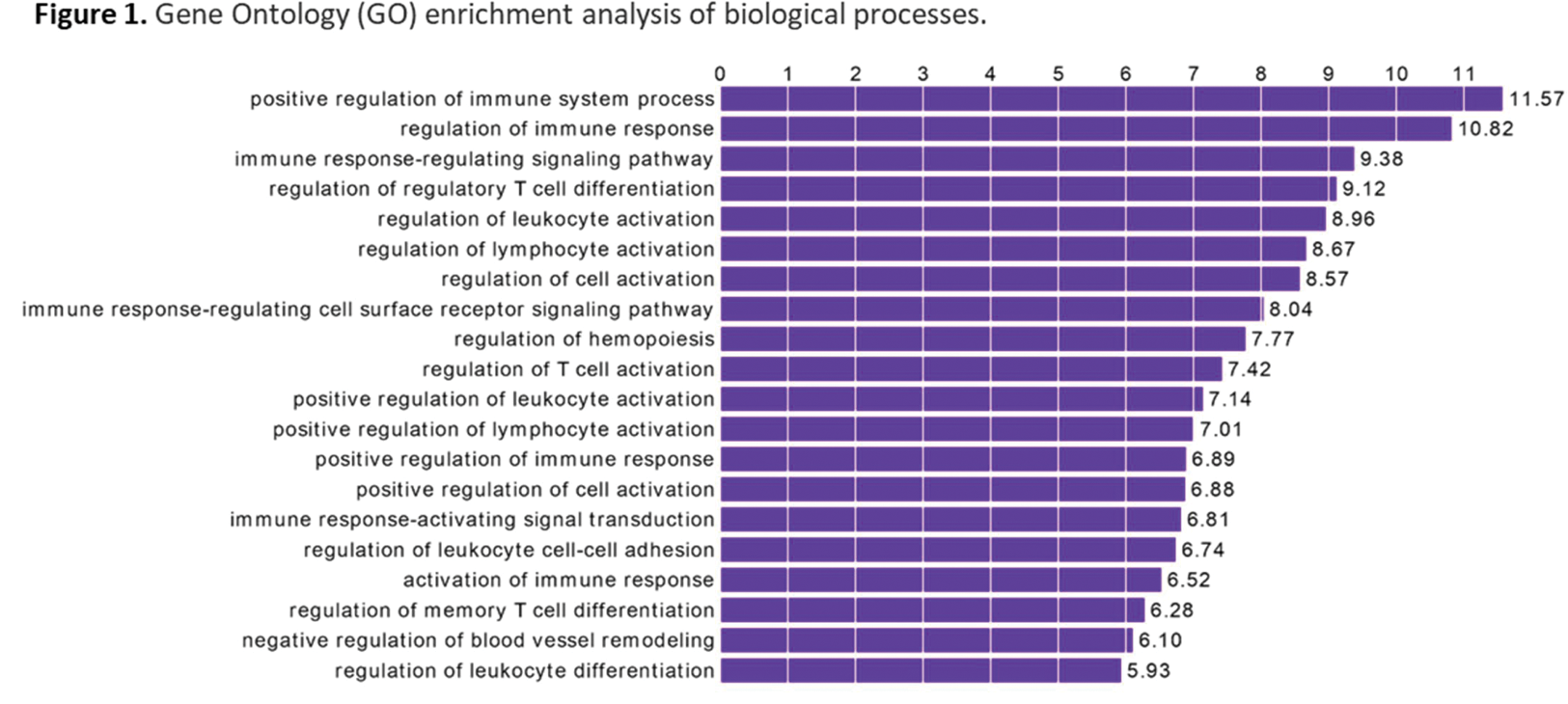
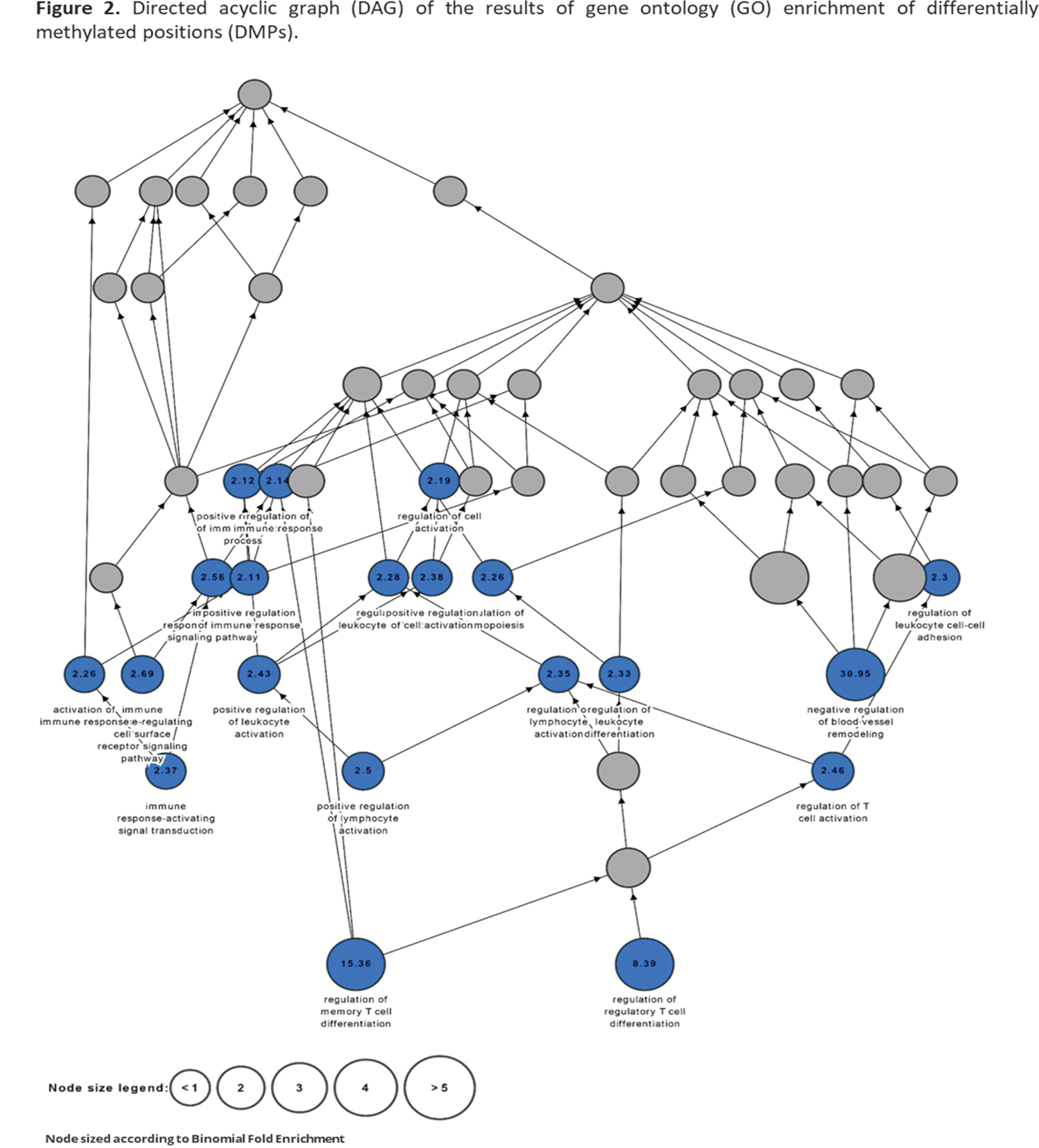
Results: Our results unveiled profound alterations in the DNA methylation profiles of B-cells between patients with IgAV and healthy controls. In particular, 611 differentially methylated positions (DMPs) across the whole genome were identified between these groups. Among them, 601 DMPs were located in one or more genes, whereas 6 were not associated with any gene. Interestingly, through gene ontology (GO) analysis of these data, we observed an enrichment in several biological processes of the immune response (such as regulation of leucocyte and T-lymphocyte activation and differentiation, immune response activation signal transduction, regulation of leucocyte cell-cell adhesion, and regulation of memory T-cell differentiation) (Figures 1 and 2). Moreover, an enrichment in other biological processes, including the negative regulation of blood vessel remodelling and the regulation of hemopoiesis, was disclosed in the GO analysis (Figures 1 and 2).
Conclusion: Our results confirm the relevant role of the immune system in the pathogenesis of IgAV and point to methylation profiles of B-cells as biomarkers of IgAV susceptibility.
REFERENCES: [1] Arthritis Rheum.2013;65:1-11 .
[2] Autoimmun Rev.2018;17:301-15.
[3] Kidney Int.2018;93:700-5.
[4] J Clin Invest.2009;119:1668-77 .
Acknowledgements: This research was funded by European Union FEDER funds and “Fondo de Investigaciones Sanitarias” from “Instituto de Salud Carlos III” (ISCIII, Health Ministry, Spain), grant number PI18/00042 and PI21/00042. VP-C is supported by funds of IDIVAL, grant number NVAL23/02. JCBL is a recipient of a PFIS program fellowship from the ISCIII, co-funded by the European Social Fund (`Investing in your future´), grant number FI22/00020. MSM-G is supported by funds of “Fondo de Investigaciones Sanitarias” from ISCIII, grant number PI18/00042. RL-M is a recipient of a Miguel Servet type II program fellowship from the ISCIII, co-funded by ESF (“Investing in your future”, grant number CPII21/00004.
Disclosure of Interests: Raquel López-Mejías: None declared, Verónica Pulito-Cueto: None declared, Iván Fernández Rengel: None declared, LAURA CARMEN TERRON CAMERO: None declared, Joao Batista-Liz: None declared, María Sebastián Mora-Gil: None declared, María Teresa Leonardo: None declared, Ana Peñalba: None declared, Luis Martín-Penagos: None declared, Lara Belmar-Vega: None declared, Cristina Gomez-Fernandez: None declared, Ligia Gabrie: None declared, Rafael Gálvez-Sánchez: None declared, Luis Caminal-Montero: None declared, Ana Isabel Turrión: None declared, Patricia Quiroga Colina: None declared, Esther Vicente-Rabaneda: None declared, Belén Sevilla-Pérez: None declared, José Luis Callejas: None declared, Eduardo Andrés-León: None declared, Javier Martin: None declared, Ana Márquez: None declared, Santos Castañeda: None declared, Ricardo Blanco Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, MSD, Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, MSD, Abbvie, MSD, Roche.