

Background: Primary Sjögren’s syndrome (pSS) is a heterogeneous systemic autoimmune disease marked with varied clinical manifestations and diversified treatment response, potentially due to different pathogeneses among patients.
Objectives: Our study aimed to clarify the etiology and molecular variation of the target organ, salivary glands (SGs), in pSS by stratifying them into distinguishable subgroups, which could inform treatment choices in pSS.
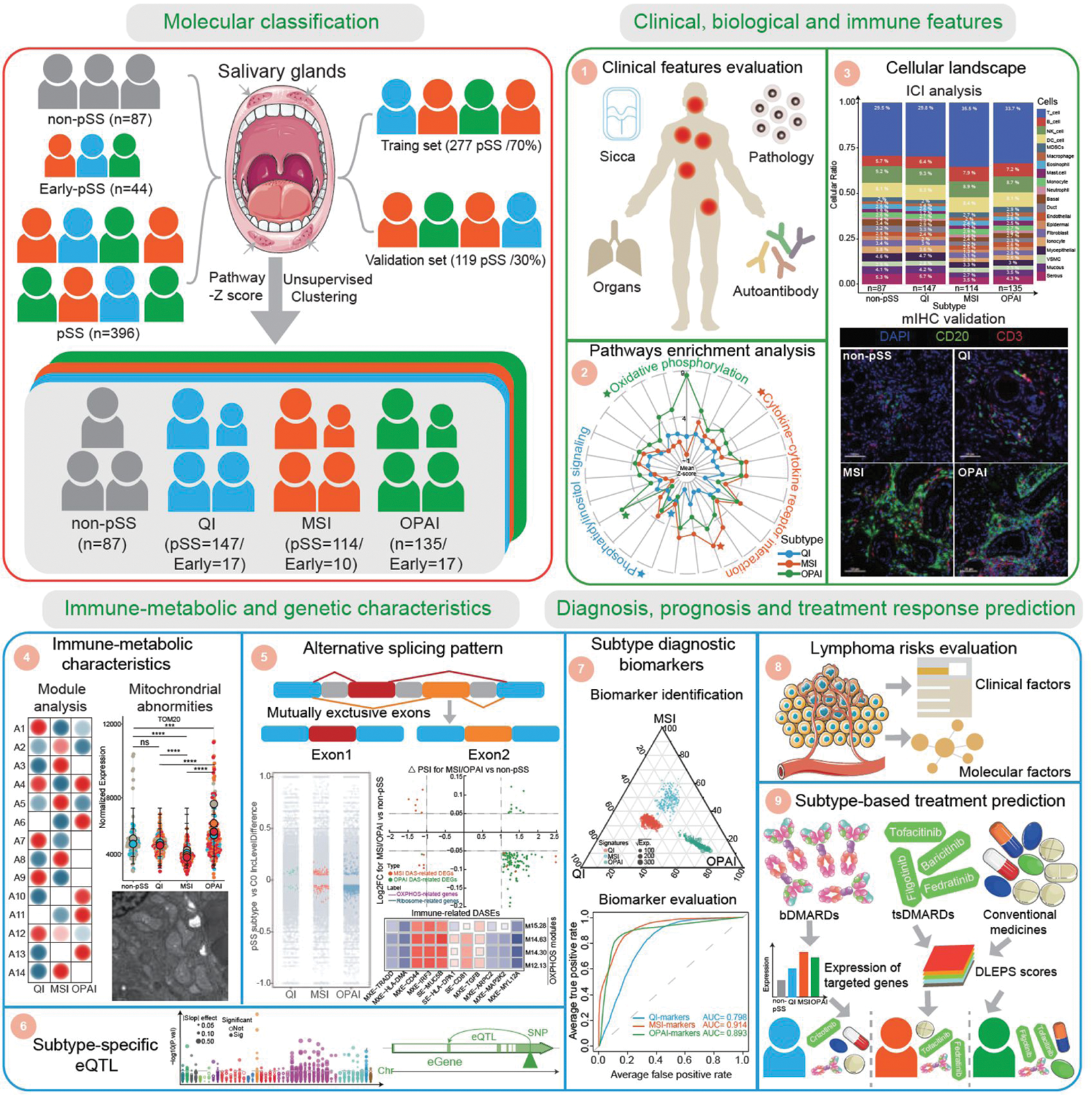
Methods: Large-scale transcriptomic profiling of SGs was conducted on 396 pSS, 87 non-pSS, and 44 early-pSS individuals from a multicenter consecutive Chinese cohort. Unsupervised clustering methods were used for molecular classification and integrated analyses were performed to elucidate comprehensive clinical and biological features, immune-metabolic and genetic characteristics. Lymphoma risk and treatment response for pSS subgroups was also predicted for pSS subtypes.
Results: In this study, we identified the following three molecular subtypes: Quiescent Immune (QI), Mitochondrial Stress Immune (MSI) and Oxidative-Phosphorylation Active Immune (OPAI). The QI subtype exhibited the lowest inflammatory and immune features among the three patient groups and had a gene expression pattern close to non-pSS. Both MSI and OPAI subtypes had significant infiltration of lymphocytes and activation of immune-related pathways. MSI subtype additionally showed prominent structural damage and dysfunction of mitochondria, which resulted in downregulation of ATP-dependent pathways, and activation of cGAS-STING signaling. However, OPAI subtype was marked with active mitochondrial oxidative phosphorylation (OXPHOS), upregulation in metabolism associated pathways and a distinct alternative splicing profile with fewer but specifically oxidative phosphorylation-associated differential alternative splicing events. In addition, we developed random forest models to discriminate these subtypes based on molecular features for potential clinical translation.
Conclusion: Overall, our findings provide a comprehensive and in-depth molecular landscape of SGs in pSS, shedding light on the disease heterogeneity and promoting the development of precise clinical intervention strategies for pSS patients.
REFERENCES: NIL.
Overview of this study. We recruited 527 participants based on respective criteria, including 87 non-pSS, 44 early-pSS, and 396 pSS. Using an unsupervised clustering approach on the transcriptomic data of SGs, we identified three distinct molecular subtypes in pSS and early-pSS. We then conducted extensive downstream analysis on their clinical and biological and immune features, immune-metabolic and genetic characteristics, and disease diagnosis, prognosis, and treatment response prediction. pSS, primary Sjogren’s syndrome; QI, Quiescent Immune; MSI, Mitochondrial Stress Immune; OPAI, Oxidative-Phosphorylation Active Immune; ICI, immune cell infiltration; mIHC, multiplex immunohistochemistry; eQTL, expression quantitative trait loci; bDMARDs, biologic disease-modifying anti-rheumatic drugs; tsDMARDs, targeted synthesis disease-modifying anti-rheumatic drugs.
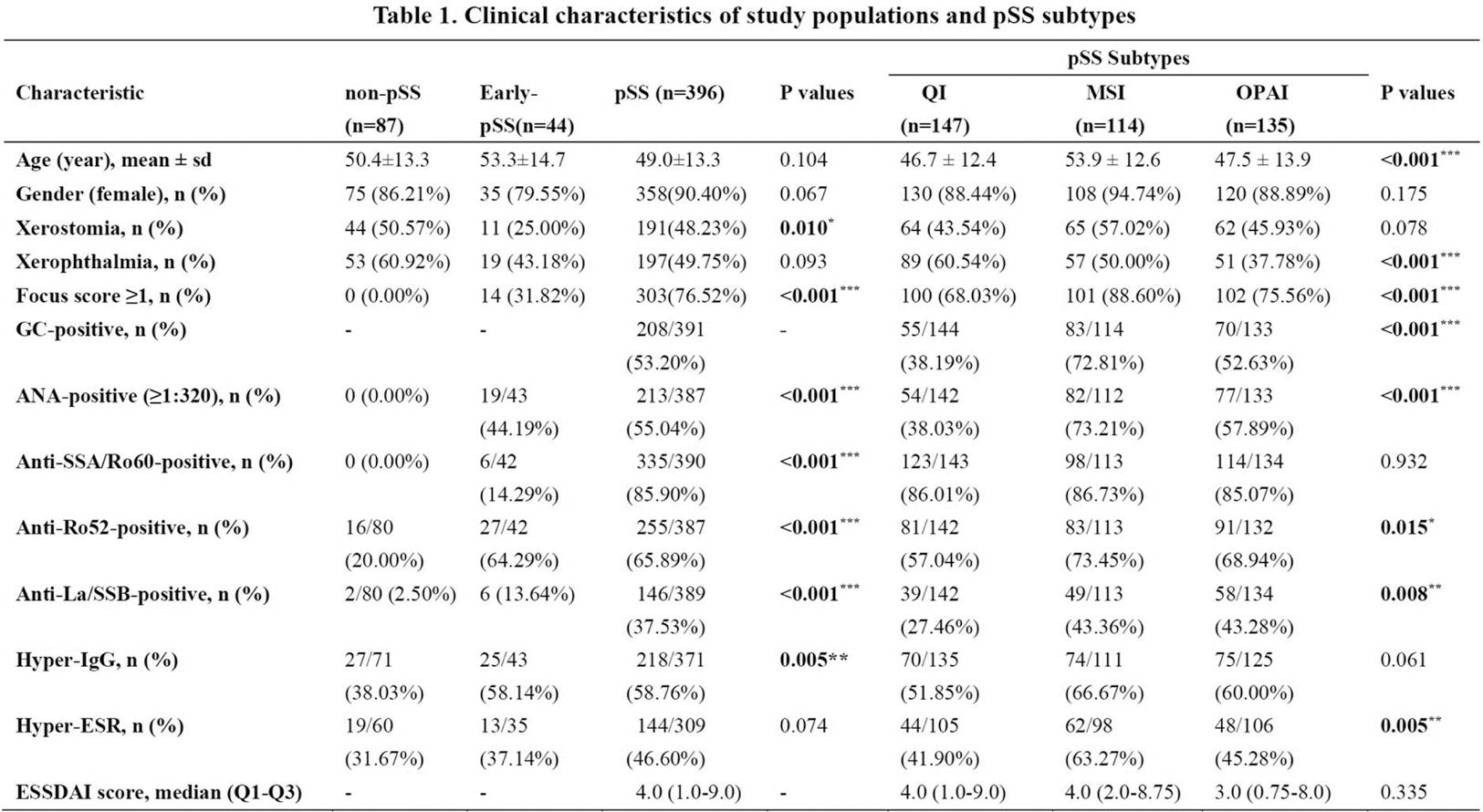
Note: GC, germinal center; OSS, ocular staining score; ANA, antinuclear antibodies; IgG, immunoglobulin G, Hyper-IgG means IgG > 16g/L; ESR, erythrocyte sedimentation rate, Hyper-ESR means ESR> 20mm/H; ESSDAI, EULAR Sjogren’s Syndrome Disease Activity Index; sd, standard deviation; QI, Quiescent Immune; MSI, Mitochondrial Stress Immune; OPAI, Oxidative-Phosphorylation Active Immune; *, p <0.05; **, p <0.01; ***, p <0.001.
Acknowledgements: NIL.
Disclosure of Interests: None declared.