

Background: Behçet’s syndrome (BS) is a chronic, recurrent systemic vasculitis that can affect multiple systems. Neuro-Behçet’s syndrome (NBS), especially parenchymal NBS(p-NBS), results in severe neurological sequelae with poor prognosis, ranging from cognitive changes to paralysis, which remains a challenging clinical issue.
Objectives: This study aimed to evaluate the efficacy and safety of infliximab (IFX), a type of anti-TNF-α agents, in treating patients with p-NBS and hopefully provide a detailed time analysis for the IFX effect timeline in NBS treatment.
Methods: We conducted this meta-analysis following the PRISMA guidelines. PubMed, Embase, Cochrane Library and Web of Science were systematically searched using the following logics: Behçet’s syndrome (all synonyms) AND Infliximab (all synonyms) AND (‘neuro’ OR ‘neurological’ OR ‘neurologic), and we also included the data from our institution in this meta-analysis. Therapeutic efficacy was evaluated by the improvement of clinical symptoms and radiological lesions, and clinical improvement was specifically defined as complete remission (CR), partial remission (PR) and no response (NR) [1,2]. We evaluated the efficacy of IFX at 3, 6, and 12 months respectively. The results were presented as forest plots with 95% confidence intervals. Besides, the details of adverse drug reactions were recorded.
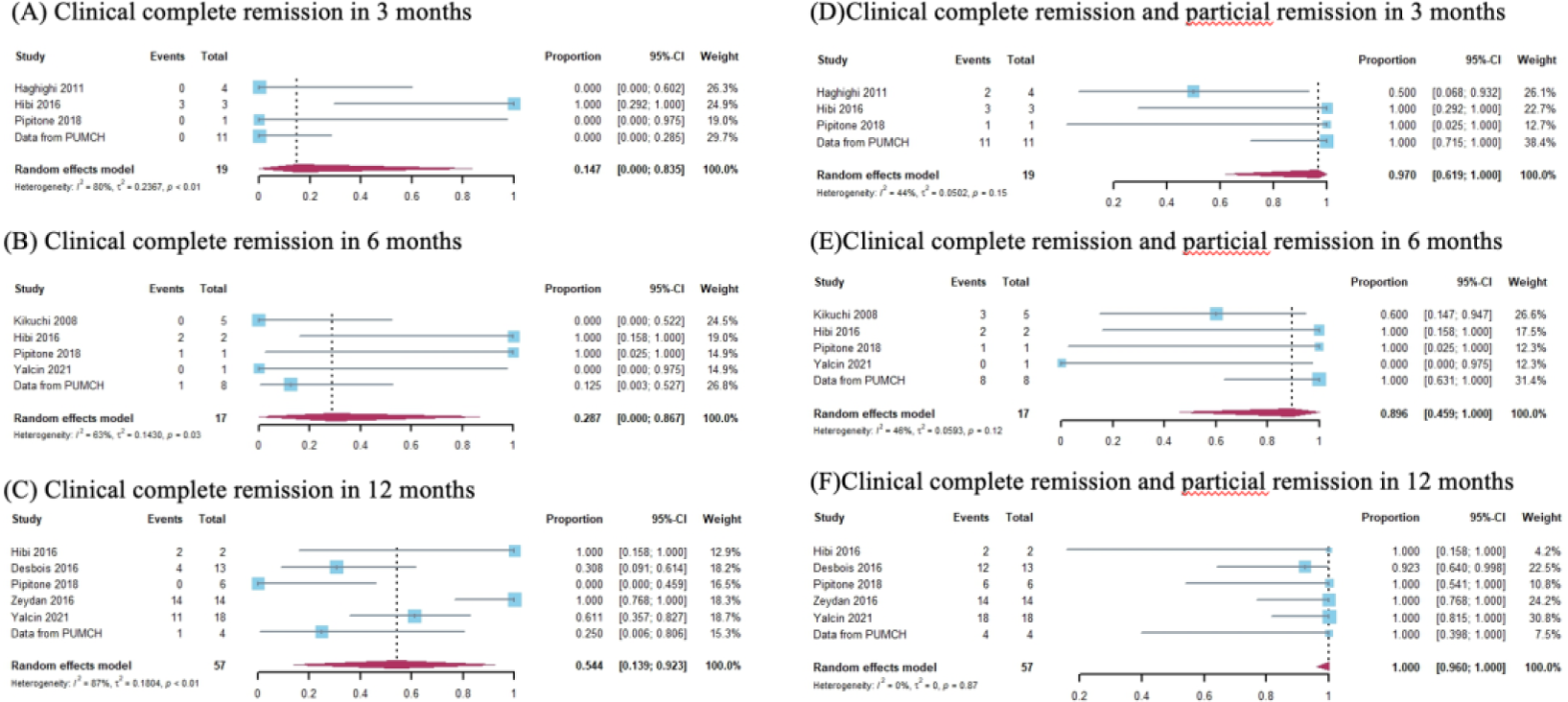
Results: 8 studies involving a total of 77 patients were identified to perform the meta-analysis by adding the results from our institution. The pooled efficacy of IFX in treating p-NBS patients were analyzed. Clinical complete remission was achieved in 14.7% (95%CI 0.00-83.5%) of patients at Month 3, 28.7% (95% CI 0.00-86.7%) of patients at Month 6, 54.4% (95%CI 13.9-92.3%) at Month 12. Clinical partial remission was achieved in 97.0% (95%CI 61.9-100%) of patients at Months 3, 89.6% (95%CI 45.9-100%) of patients at Months 6, 100.0% (95%CI 96.0-100%) at Months 12. MRI improvement was achieved in 100.0% (95%CI 89.7-100%) of patients at Month 3, 89.1% (95% CI 26.3-100%) of patients at Month 6, 99.5% (95% CI 96.0-100%) at Month 12. The time-phase analysis indicated that IFX exerted its effect within 3 months and was sustained during the long term. Safety information was reported in 16 patients. Among them, 9% of patients experienced severe adverse events (AEs), no AE-related deaths were reported.
Conclusion: IFX is an effective treatment option for p-NBS, and the incidence of severe AEs was acceptable. A comprehensive evaluation should be performed after three months of medication to determine the need for adjustments in subsequent therapy. Further well-designed, large-scale RCTs are needed to verify our findings.
REFERENCES: [1] Hibi T, Hirohata S, Kikuchi H, et al. Infliximab therapy for intestinal, neurological, and vascular involvement in Behcet disease: Efficacy, safety, and pharmacokinetics in a multicenter, prospective, open-label, single-arm phase 3 study. Medicine (Baltimore) 2016;95:e3863.
[2] Pipitone N, Olivieri I, Padula A, et al. Infliximab for the treatment of neuro-Behçet’s disease: A case series and review of the literature. Arthritis Care and Research 2008;59:285-90. (Article).
Results of pooled proportions of clinical complete remission in patients treated by IFX agents in 3 months (A), 6 months (B), and 12 months (C). Results of pooled proportions of clinical complete remission and partial remission in patients treated by IFX agents in 3 months (D), 6 months (E), and 12 months (F). Data from PUMCH was carried out in our institution and was not published before.
Acknowledgements: We thank all the participants and their families.
Disclosure of Interests: None declared.