

Background: Spondyloarthritis (SpA) is known to be associated with subclinical gut inflammation, emphasizing the existence of a gut-joint axis. However, the links between gut dysbiosis and intestinal inflammation remain unclear, particularly regarding the mechanistic sequence. A growing aspect of gut homeostasis involves VIPergic enteric innervation, influencing immune responses and controlling the expression of fucosyltransferase (FUT) enzymes crucial for fucosylation and microbiota balance. In axial SpA (AxSpA), there is evidence of HLA–B27–restricted T cells demonstrating autoreactivity against Vasoactive Intestinal Peptide Receptor 1 (VIP1R) and other neuron-associated antigens. The functional consequences of this autoreactivity within AxSpA are not yet fully explored, indicating a potential role for neuroinflammatory processes in the pathogenesis of AxSpA.
Objectives: The primary objective of this study is to determine the presence and extent of neuroinflammation and its relationship to dysbiosis and gut inflammation in AxSpA patients.
Methods: We analyzed the expression of key neurotransmitter and neuron-associated molecules: glial-derived neurotrophic factors ( GDNF ), GDNF Family Receptor Alpha ( GFRA1 ), neurturin ( NRTN ), Vasoactive intestinal peptide ( VIP) and VIP receptor (VIPR ) in ileal tissues from treatment-naïve AxSpA patients satisfying the ASAS criteria and healthy controls (HC), each group of 10 individuals. RT-PCR and immunohistochemistry were employed to assess the expression levels. Neuroinflammatory markers were examined in SKG mice treated with curdlan, a pre-clinical model for SpA and ileitis (n=8). Immunofluorescence microscopy and flow cytometry also evaluated alterations in intestinal fucosylation (UEA-1 staining) and co-localization of nerves and immune cells. SKG mice in germ-free conditions were used to investigate the impact of microbiota.
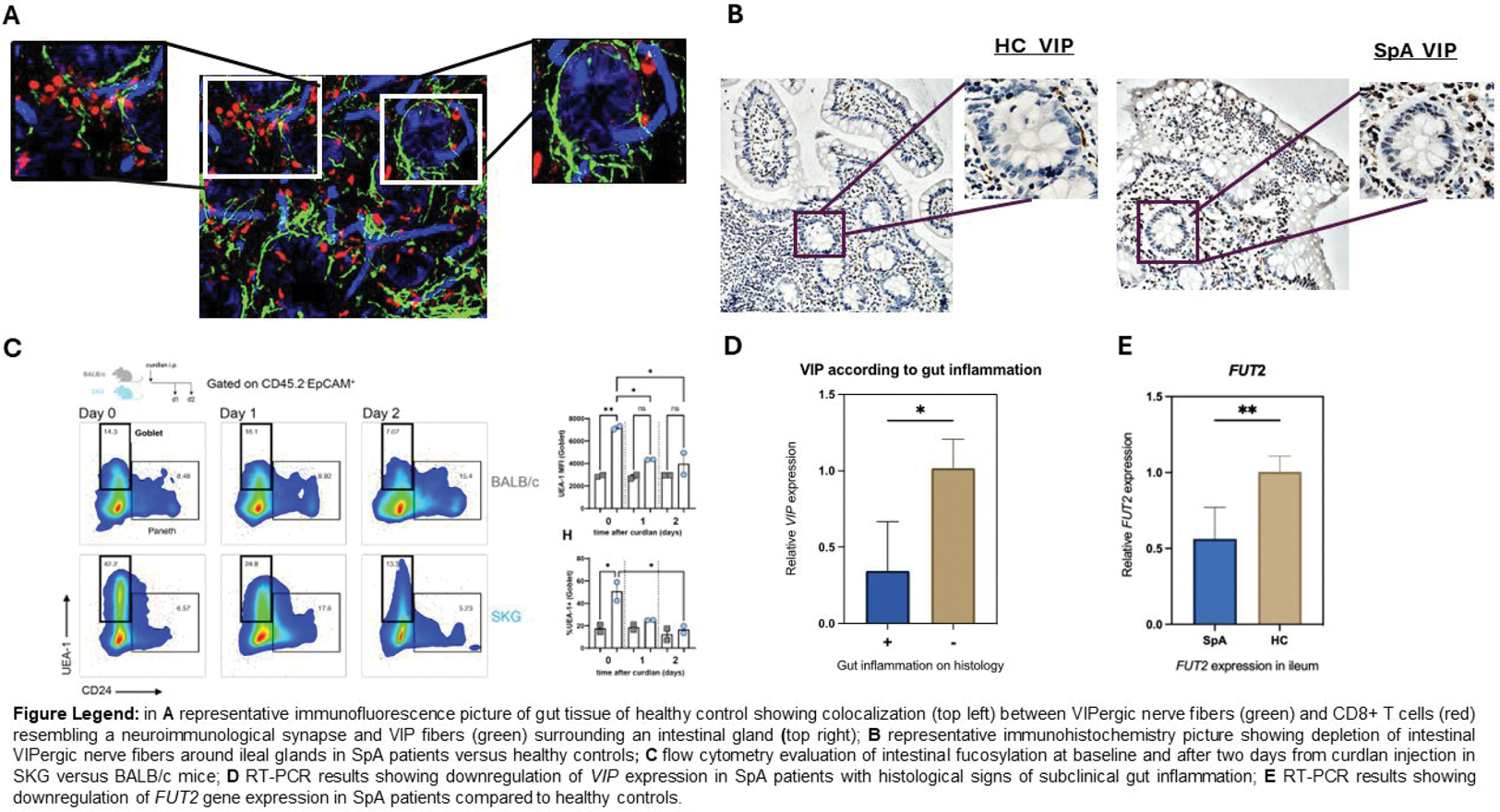
Results: In the ileum of AxSpA patients, GDNF , GFRA1 , NRTN , VIP, and VIPR expression was significantly reduced compared to controls (all p <0.05), with more pronounced downregulation in males and patients with histological signs of subclinical gut inflammation ( r -0.82, p <0.02). Immunofluorescence microscopy revealed that VIPergic nerve fibers, in the healthy gut, surrounded intestinal crypts and exhibited colocalization with CD8 + T cells, resembling a neuro-immunological synapse. Although the quantity of VIP-producing mononuclear cells was similar in AxSpA and controls, VIPergic fibers were significantly reduced in the gut of AxSpA. In the ileum, Vip and Vpac2 were significantly higher in untreated SKG than control BALB/c mice. Vip and Gdnf significantly decreased in SKG ileum seven days post-curdlan while remaining unchanged in control BALB/c mice. Intriguingly, this reduction was microbiota-dependent, as germ-free conditions abrogated it. Since fucosylation underpins microbiota homeostasis, we examined Fut2 and intestinal fucosylation in AxSpA patients. Together with reduced VIP expression, we observed decreased fucosylation in SpA patients, underscored by lower FUT2 expression (p<0.05). In SKG mice, the administration of curdlan resulted at day 1 and day 2 decrease in fucosylated protein in epithelial cells as shown by UEA-1 binding in flow cytometry followed by an increase in Fut2 expression later point (day 7).
Conclusion: This study reveals a marked depletion of intestinal nerve fibers and neurotrophic factors in SpA patients and a microbiota-dependent disturbance of VIP regulation in ileitis-prone SKG mice, with reduced Vip correlating with intestinal stress and permeability. Upregulation of Fut2 may be induced by microbial translocation and local inflammation post-curdlan in SKG mice. These findings suggest a bidirectional relationship where neuroinflammatory marker expression is influenced by the gut microbiota, and altered fucosylation perpetuates ileal dysbiosis. This new evidence may also elucidate the functional consequences of autoreactivity against neuronal markers and VIP receptors previously observed in SpA.
REFERENCES: [1] Gracey E et al. Nat Rev Rheumatol. 2020.
[2] Lei C, et. al. Cell Host Microbe. 2022.
Acknowledgements: NIL.
Disclosure of Interests: None declared.