

Background: Bacterial translocation across the gut barrier has been implicated in autoimmune diseases, including systemic lupus erythematosus (SLE). However, it remains unclear how translocated bacteria contribute to the development of autoimmunity.
Objectives: The present study aimed to elucidate the role of gut commensal translocation in the context of molecular mimicry, by utilizing lupus model mice and blood samples from untreated patients with SLE.
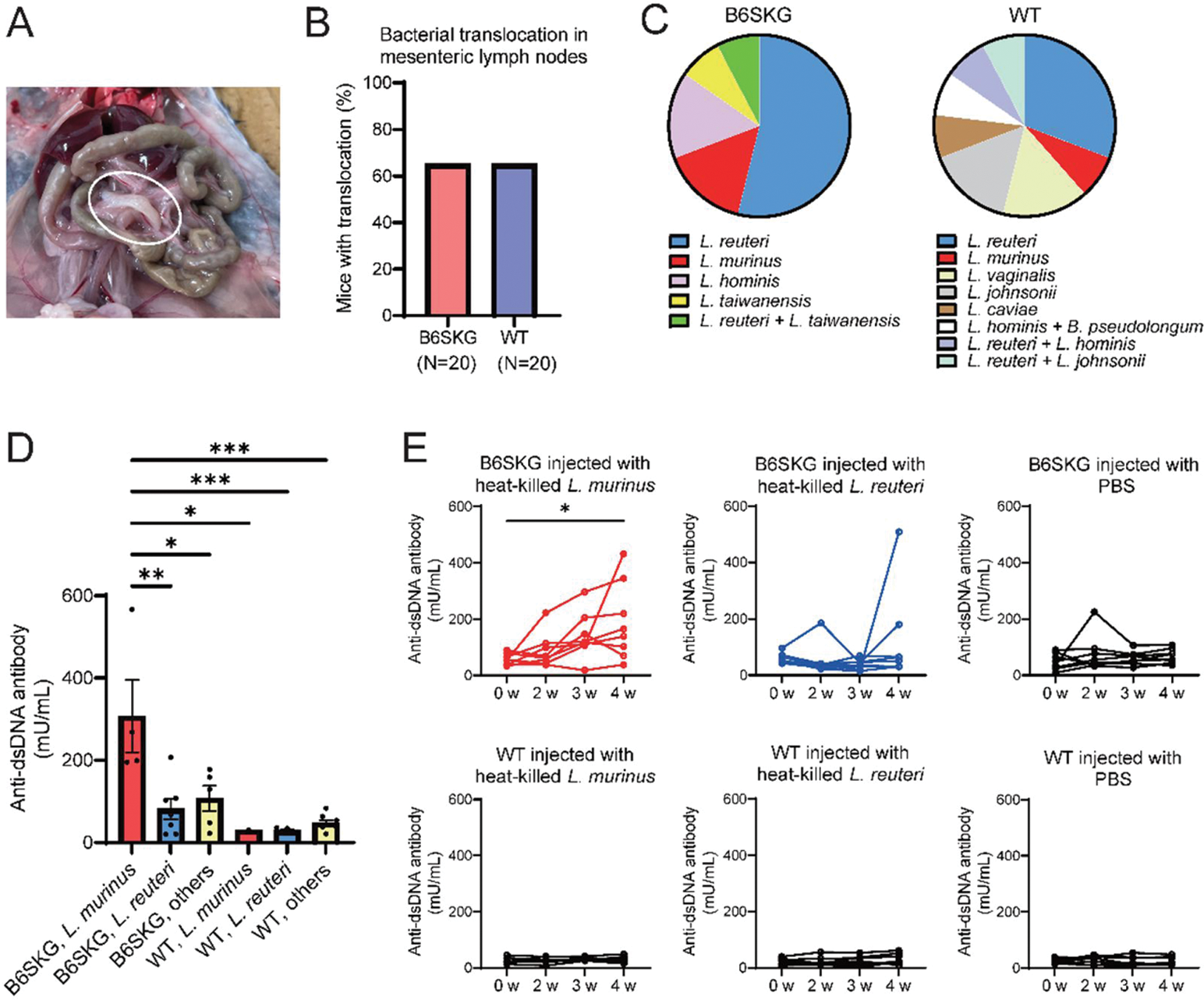
Methods: We have previously generated C57BL/6 Zap70skg/skg (B6SKG) mice, a lupus model characterized by expanded autoreactive T cells and gut dysbiosis. C57BL/6 (wild-type [WT]) mice were used as controls. All mice were maintained under specific pathogen-free conditions at Kyoto University. Bacterial translocation was evaluated by non-selective culture of mesenteric lymph nodes (MLNs). The pathogenic potency of cultured bacteria and its antigen was investigated by in vivo experiments, ELISA, western blotting and epitope mapping.
Results: The rates of bacterial translocation were same between B6SKG and WT mice. The profiling of cultured bacteria in MLNs demonstrated that Lactobacillus murinus , known as a gut symbiont in rodents, was enriched in B6SKG mice with increased anti-dsDNA antibody levels. The injection of heat-killed L. murinus replicated the autoantibody production in B6SKG mice. A protein band of L. murinus with cross-reactivity to anti-dsDNA antibodies was analyzed by mass spectrometry, and identified as peptide ATP-binding cassette transporter (ABCT). Recombinant L. murinus ABCT was generated, and its cross-reactivity was confirmed by in-vivo immunization and ELISA inhibition assay. In addition, anti-ABCT antibody levels were significantly correlated with anti-dsDNA antibody levels in B6SKG mice. Notably, SLE patients also showed elevated anti-ABCT antibody levels, and epitope mapping of L. murinus ABCT revealed common binding sites between mice and humans. Part of the binding sequences was predicted as MHC class II-restricted epitopes, and located on the protein’s surface in the three-dimensional model structure.
Conclusion: The translocation of a gut symbiont with its mimicry antigen contributes to lupus autoantibody production. Bacterial translocation and commensal-induced molecular mimicry may play pathogenic roles in hosts predisposed to autoimmunity.
( A ) Representative images of mesenteric lymph nodes in mice after sterile laparotomy. ( B ) Total percentage of bacterial translocation in B6SKG and wild-type (WT) mice. ( C ) Profile of translocating bacterial species in mesenteric lymph nodes. ( D ) IgG anti-dsDNA antibody levels measured via ELISA. Mice were grouped based on the translocating bacteria: L. murinus , L. reuteri , and others. ( E ) Time-course variations in anti-dsDNA antibody levels in mice injected with heat-killed bacteria.
( A ) Western blotting of recombinant L. murinus ATP-binding cassette transporter (ABCT), probed with mouse sera. ( B ) A scatterplot of the correlation between anti-ABCT and anti-dsDNA antibody levels, measured via ELISA. ( C ) Anti-ABCT antibody levels in B6SKG mice and humans. ( D ) Epitope mapping of L. murinus ABCT. 15-mer overlapping peptides (shift by three amino acids) were arrayed on membranes and probed with sera from B6SKG mouse and SLE patients. ( E ) Sequence alignment with predicted MHC class II epitopes. The F19-25 sequences (referenced in D ) are aligned with H2-IAb-restricted epitopes (top) and HLA-DRB-restricted epitopes (bottom).The identified epitopes are highlighted in green, orange, and magenta. ( F ) Identified epitopes of L. murinus ABCT, illustrated on the three-dimensional model structure.

REFERENCES: NIL.
Acknowledgements: We would like to sincerely thank all those who have been involved for their valuable contributions.
Disclosure of Interests: None declared.