

Background: Axial spondyloarthritis (axSpA) is a chronic inflammatory disorder predominantly affecting axial joints, leading to persistent back pain and stiffness. In cases where patients continue to experience active symptoms despite receiving maximum doses of nonsteroidal anti-inflammatory drug therapy, TNF-α inhibitors (TNFi) are suggested as a second-line treatment. However, the response to TNFi is highly heterogeneous, with approximately 30% of patients being non-responsive. Yet, there is a notable absence of studies investigating prognostic markers for predicting the response to TNFi in individuals with axSpA, especially in genetic and transcriptional levels.
Objectives: We aimed to identify cell types and cell-type specific transcriptional markers associated with the response to TNFi in patients with axSpA.
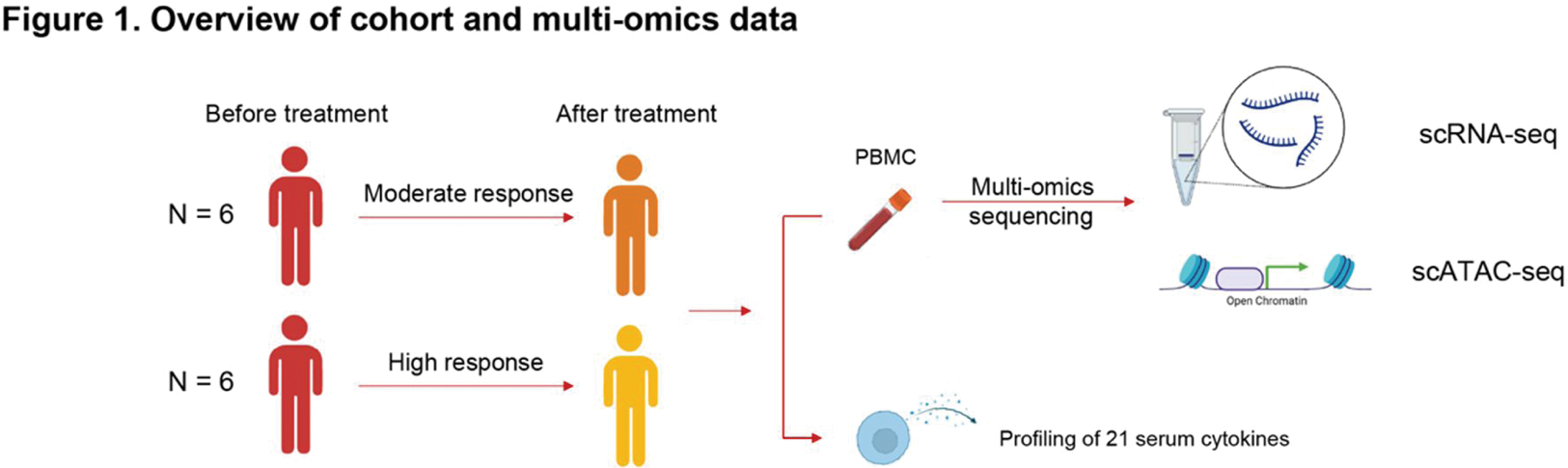
Methods: A prospective cohort of twelve biologics-naïve male patients with axSpA treated with TNFi at Samsung Medical Center underwent matched single-cell RNA sequencing (scRNA-seq) and single-cell assay for transposase accessible chromatin sequencing (scATAC-seq) on peripheral blood mononuclear cell (PBMC) samples collected before and three months after treatment (Figure 1). The cohort was categorized into high responders (patients meeting major improvement criteria, ΔASDAS ≥ 2.0) and moderate responders (patients meeting clinically important improvement criteria, 1.1 ≤ ΔASDAS < 2.0) based on the Ankylosing Spondylitis Disease Activity Score (ASDAS) response criteria.
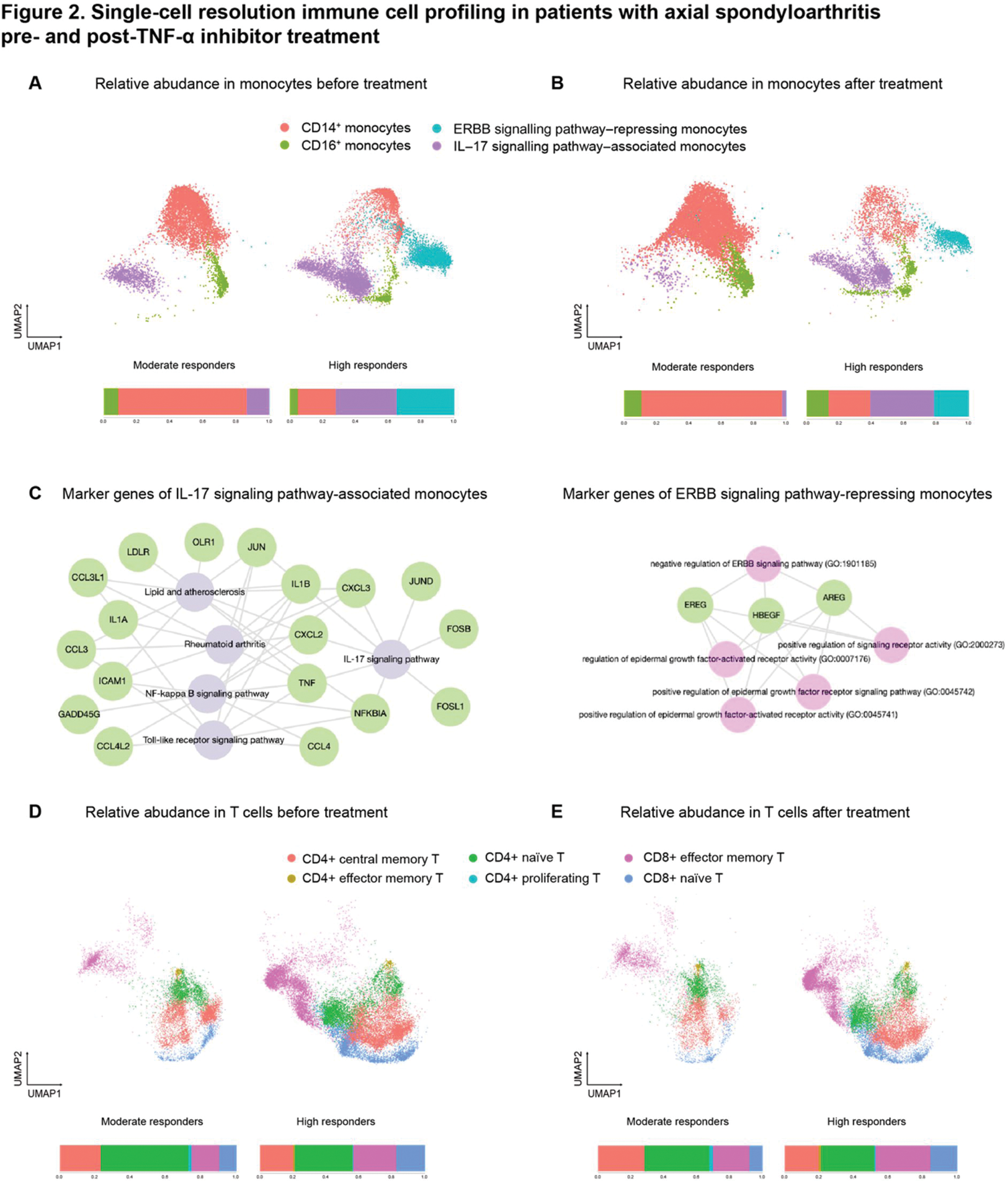
Results: In terms of innate immunity, two distinct subsets of CD14+ monocytes linked to TNFi response were identified. Prior to treatment, high responders showed significantly higher relative abundance of IL-17 signaling pathway-associated CD14+ monocytes (Figure 2A). The genetic intersection between IL-17 and TNF signaling pathways suggests a potential influence on treatment response (Figure 2C, left panel). Similarly, CD14+ monocytes associated with the repression of the ERBB signaling pathway (Figure 2C, right panel) were more abundant in high responders before treatment (Figure 2A), with their proportion decreasing after the treatment in this group (Figure 2B). The inverse correlation between the expression of an ERBB family protein and TNF-α implies a potential impact on TNFi response. In adaptive immunity, high responders showed an elevated pre-treatment fraction of CD8+ effector memory cells associated with TNFi response (Figure 2D). After the treatment, the fraction of CD8+ effector memory cell was even more increased (Figure 2E). Analysis of differentially expressed genes and accessible regions revealed up-regulation of NF-kappa B signaling pathway genes (CCL4L2 and TNFAIP3) and NOD-like receptor signaling pathway genes (NAMPT and TNFAIP3) in CD14+ monocytes before treatment. In CD8+ T lymphocytes, up-regulated pathways included IL-17 signaling (NFKBIA, JUN, JUND, TNFAIP3, FOSB, FOS), osteoclast differentiation (NFKBIA, JUN, JUND, FOSB, FOS), Toll-like receptor signaling (NFKBIA, JUN, CCL4, FOS), and TNF signaling (NFKBIA, JUN, TNFAIP3, FOS). Overall, high responders exhibited a shift towards pro-inflammatory states, potentially contributing to increased treatment response. Serum cytokine profiling demonstrated significant associations of IL-8, IL-6, and TIMP-1 with TNFi treatment responses, suggesting these cytokines may serve as potential biomarkers for predicting the response to TNFi.
Conclusion: This study provides insights into potential prognostic markers for TNFi response in axSpA patients, highlighting specific cell types and transcriptional markers associated with treatment outcomes. The identification of CD14+ monocyte subsets and relevant signaling pathways offers a foundation for further research and the development of personalized therapeutic strategies in the management of axSpA. Additionally, the association of serum cytokines with treatment responses suggests their potential utility as biomarkers in assessing and predicting TNFi outcomes in individuals with axSpA.
REFERENCES: NIL.
Acknowledgements: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2021R1F1A1062148 and 2022R1F1A1071471).
Disclosure of Interests: None declared.