

Background: An overreactive immunoprofile note as “IFN-signature”, in which often IFNγ signal deregulation precedes and triggers IFNα aberrant profile associated with clinical manifestation is involved in the pathogenesis of autoimmune diseases, i.e., Systemic Lupus Erythematosus (SLE) [1]. The chemokine CXCL10 acts at IFNγ/IFNα signaling intersection and plays an early role in initiation and progression of autoimmune response [2]. The peroxisome proliferator-activated receptor (PPAR)β/δ has emerged to function as a “molecular brake” in autoimmunity limiting self-overreaction [3].
Objectives: This study investigates the impact of PPARβ/δ activation by its specific ligand carbaprostacylin (CARB) on CXCL10 secretion and expression in human CD4+ T cells and dendritic cells (DCs), both widely engaged in autoimmune disease pathogenesis. We also evaluated TNFα, IFNγ and IL-10, the latter one playing a pivotal role as inflammation counterregulatory molecule. For comparison, PPARγ agonist rosiglitazone (RGZ) was also used.
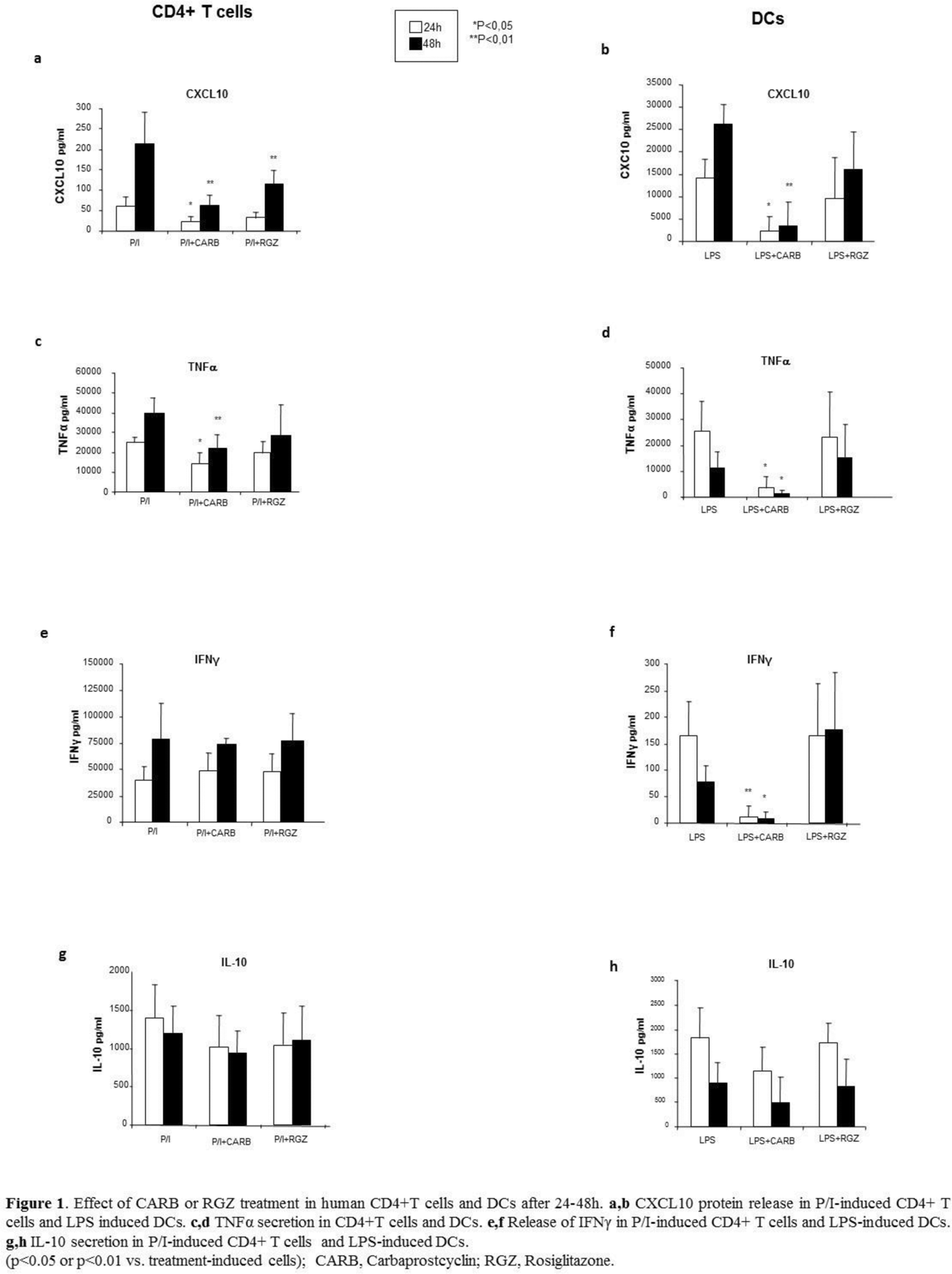
Methods: Human CD4+ T cells and DCs were obtained from healthy donors and activated for 24-48h with P/I and LPS, respectively, as previously described [4]. Cell supernatants were tested by Luminex for CXCL10, TNFα, IFNγ, and IL-10 level after the treatment with CARB or RGZ [10µM and 5µM near-therapy doses, respectively] at both time points. Specific mRNA for the same analyte pattern was investigated by Real Time q-PCR in extracts from cells in the same experimental conditions after 24h. Cells maintained in media w/ vehicle w/o stimuli were used as control. Statistical analysis was performed with SPSS 12.0 software package; p ≤ 0.05 was considered significant.
Results: CXCL10 secretion was significantly reduced in CD4+ T cells and DCs 24 and 48h after CARB vs. P/I- or LPS-induced treatment (p<0.05 or p<0.01). While CARB significantly decreased TNFα release induced in CD4+T cells and DCs at both time points, IFNγ secretion decreased only in DCs (p<0.05 or p<0.01 vs. induced secretion). mRNA expression was found significantly reduced (p<0.01), except for TNFα in CD4+ T cells. CARB did not affect induced IL-10 secretion in CD4+ T cells and DCs, albeit the specific mRNA was up- or downregulated, respectively. RGZ never modified protein release and mRNA expression, except for CXCL10 release reduced after 48h in CD4+ T cells (p<0.01) (Figure 1 a-h).
Conclusion: The selective targeting of PPARβ/δ in human CD4+ T cells and DCs significantly downregulated CXCL10, TNFα and IFNγ, and might explain at least in part its role as a molecular brake in autoimmunity. The reduction of CXCL10 might be particularly relevant due to its early role at IFNγ/IFNα signaling intersection. In this scenario, re-thinking of PPARβ/δ agonists for therapeutic purposes in autoimmunity management might be suggested.
REFERENCES: [1] Spinelli et al. Autoimm Rev. 2023 doi: 10.1016/j.autrev.2023.103412.
[2] Lee et al. Autoimmunity Rev 2009 doi:10.1016/j.autrev.2008.12.002.
[3] Dunn et al. 2010 J of exp med doi: 10.1084/jem.20091663.
[4] Sottili et al. JEI 2022 doi: 10.1007/s40618-021-01621-5.
Acknowledgements: NIL.
Disclosure of Interests: None declared.