

Background: Lupus nephritis (LN) is associated with high systemic type I interferon (IFN) activity; over 90% of patients with active class III/IV LN have high IFN gene signature (IFNGS) scores in blood.[1,2] Type I IFN-dependent gene expression has also been observed in the tubular compartment of the kidney,[3] but less is known about its expression in the glomerular compartment.[4] Thus, further evidence is needed to support the association between type I IFN activity in these compartments and across various kidney diseases.
Objectives: To evaluate IFNGS expression in the kidney glomeruli and tubules of patients with LN and in a broader set of chronic kidney diseases (CKDs) using publicly available datasets.
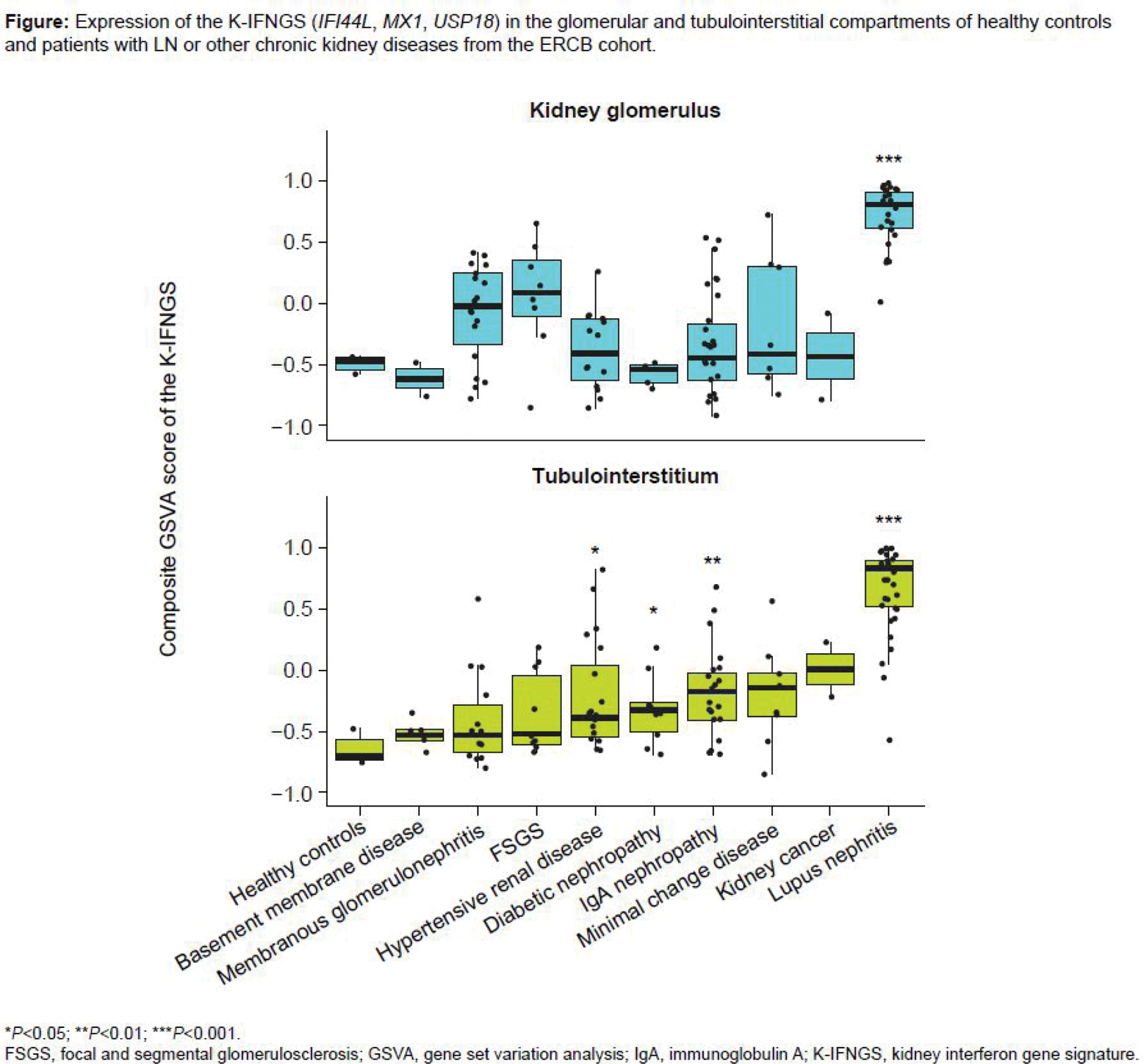
Methods: We ran a Gene Set Enrichment Analysis for the Human Gene Set “HALLMARK_INTERFERON_ALPHA_RESPONSE” (ID: M5911) in two “LN vs Control” public transcriptomic datasets, GSE32591 and GSE98422.[5,6] The top three common genes upregulated in LN vs controls in both training datasets were combined to generate a type I IFNGS for the kidneys (K_IFNGS). The Gene Set Variation Analysis (GSVA) composite score for the K_IFNGS was evaluated in a third LN dataset (ERCB), which included LN and other CKDs,[7] and in an LN single-cell RNA sequencing dataset.[3] The GSVA score was compared in patients with kidney diseases vs healthy individuals using Wilcoxon signed-rank tests (with Benjamini-Hochberg P -value adjustment).
Results: The “HALLMARK_INTERFERON_ALPHA_RESPONSE” gene signature was upregulated in kidney biopsies of patients with LN compared with healthy controls in both GSE32591 and GSE98422 datasets; the top three common genes upregulated in LN in both training datasets were IFI44L (log fold change=3.83 and 2.29 in GSE32591 and GSE98422, respectively), MX1 (3.21 and 1.75), and USP18 (2.31 and 1.08) (all adjusted P -values <0.01). These genes formed the K_IFNGS, which was validated in the ERCB dataset, showing a consistent, significant upregulation in both the tubulointerstitial and the glomerular kidney compartments of patients with LN compared with healthy individuals ( P <0.001 in both compartments; Figure 1). Other CKDs had numerically higher GSVA scores in the kidney glomerulus, and there was significant ( P <0.05) upregulation of the K_IFNGS in the tubulointerstitium for hypertensive renal disease, diabetic nephropathy, and IgA nephropathy vs healthy controls (Figure 1). At the single cell level, IFI44L and MX1 were upregulated in multiple cell populations of the kidney in LN vs controls.
Conclusion: Our study identified a strong upregulation of a novel kidney IFNGS in the tubulointerstitium and glomerulus of patients with LN, and in the tubulointerstitium of patients with other CKDs, providing additional evidence that dysregulated type I IFN may modulate disease pathogenesis in these compartments. In a randomized trial in patients with LN, type I IFN signaling blockade with anifrolumab strongly inhibited a 21-gene IFNGS in whole blood that included the 3 genes of the K_IFNGS. 1 Assuming a systemic treatment effect, anifrolumab could inhibit the K_IFNGS in the kidney, potentially interfering with the immunopathogenesis of LN. Further investigation is needed to determine if this effect is observed and if suppression of the K_IFNGS in kidneys could translate to clinical benefit of anifrolumab in patients with LN.
REFERENCES: [1] Jayne D, et al. Annals Rheum Dis . 2022;81:496–506.
[2] Feng X, et al. Arthritis Rheum . 2006;54:2951–62.
[3] Der E, et al. Nat Immunol . 2019;20:915–27.
[4] Iwamoto T, et al. J Rheumatol . 2022;49:388–97.
[5] Berthier CC, et al. J Immunol. 2012;189:988–1001.
[6] Liao Z, et al. Front Immunol. 2019;10:975.
[7] Lindenmeyer MT, et al. ERCB-Nephromine-EURenOmics Database. 2014.
Acknowledgements: This study was sponsored by AstraZeneca. Writing assistance by Sofia Fernandes, PhD, and Heather Hultzapple, PharmD, of JK Associates Inc., part of Avalere Health.
Disclosure of Interests: Nicola Ferrari Shareholder: Astrazeneca, Employee: Astrazeneca, Eduardo Mysler Speakers bureau: Pfizer, AbbVie, Sanofi, Novartis, Amgen, Astra, Janssen, Consultant of: Hi Bio, Alpine Immunology, GSK, AbbVie, Pfizer, Janssen, Grant/research support: Novartis, Roche, AbbVie, Pfizer, Lilly, Janssen, James Tumlin: None declared, Brad H. Rovin Consultant of: Alexion Pharmaceuticals, Alpine Immunosciences, Astra Zeneca, Aurinia Pharmaceuticals Inc., BioCryst, Biogen Idec, BioMarin, Bristol-Myers Squibb, Callidatis, Clinical Education Alliance, CTI - Clinical Trial Services, Inc./Neovii Biotech, Exagen, Galapagos, Gilead, GlasxoSmithKline, HIBio, Horizon, Janssen, Kezar Life Sciences, Kyverna, Lilly, LuCIN, Lupus Foundation of America, MorphoSys, Neovii, Nephronet, NIH, Novartis, Omeros, Ono Pharmaceuticals, Inc., Otsuka, Pfizer, PureSpring, Q23 Bio, Resonance, RILITE Foundation, Roche/Genentech, Inc, Sana, TOME Biosciences, Travere Therapeutics, Inc., University of Minnesota, Vera Therapeutics, Vistera, Grant/research support: AMPEL BioSolutions, LLC, Artiva, Astra Zeneca, Aurinia Pharmaceuticals Inc., ChemoCentryx, Inc., EMD-Serono Inc., Equilium, GlasxoSmithKline, IQVIA, Bristol-Myers Squibb, Callidatis, LuCIN, MorphoSys, NIH, Omeros, Parexel Intl Corp, Roche/Genentech, Inc, Sana, Travere Therapeutics, Inc., Hans-Joachim Anders Speakers bureau: AstraZeneca, Bayer, GSK, Novartis. Otsuka, Roche, Consultant: AstraZeneca, Otsuka, Bayer, GSK, Novartis, Grant/research support: Boehringer Ingelheim, Eszter Csomor Shareholder: AZ full time employee and shareholder, Employee: AZ full time employee, Patrick G. Gavin Employee: Astrazeneca, Adam Platt Shareholder: Astrazeneca, Employee: AstraZeneca, Kevin Woollard Shareholder: AstraZeneca, Employee: Current employee of AstraZeneca, Rasmus Agren Employee at AstraZeneca, Have been consulting for Novo Nordisk., Catharina Lindholm Shareholder: AstraZeneca, Employee: AstraZeneca.