

Background: DMARDS and biologics are the cornerstone of therapy of autoimmunity and GVHD. However, a sizeable proportion of patients remains still with unsatisfactory disease control. Gliding on the relative success in Cancer, cell therapy is now being developed for autoimmunity. Current efforts for CarTreg are mostly focused on engineering patient’s Treg specific for a single antigen, deemed relevant for the etiopathogenesis of the disease, These approaches have inherent imitations.
Objectives: To engineer and test in vitro and in humanised mouse models of GVHD and autoimmune disease CarTreg which: i) home to microenvironments where inflammatory cytokines are present, and ii) are induced by such inflammatory cytokines in producing locally tolerogenic cytokines, thus inducing immune tolerance, with an effective clinical control based on the induction of active immune tolerance.
Methods: We have engineered a first in class lentivirus (PanTreg herein) which: a) binds specifically pro-inflammatory cytokines such as TNF and IFN; b) upon binding, induces expression of FoxP3, TGFb and IL-10 in transfected human polyclonal T cells (Gen1 PanTreg). We have developed and validated this construct in vitro and in vivo in a humanised model of GVHD.
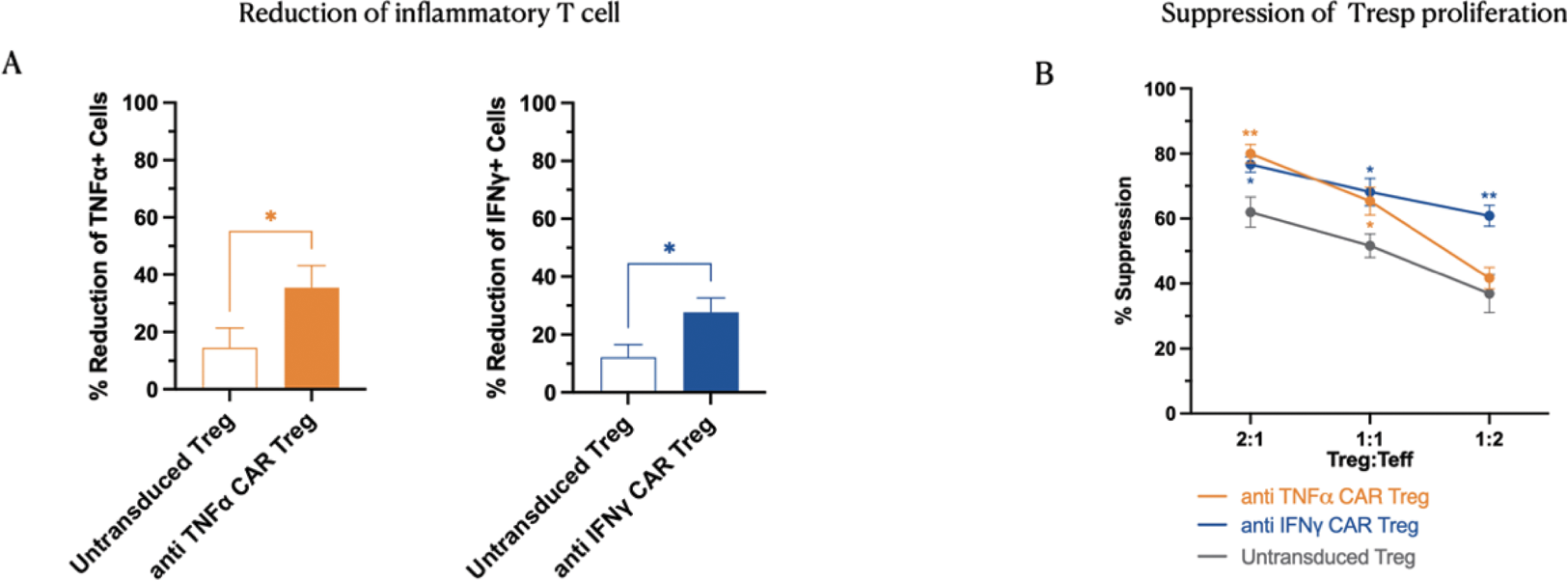
Results: PanTreg are effective in reducing human T effector function in vitro: We compared polyclonal untransduced Treg and PanTreg from the same individuals (n=5) for their ability to reduce Teff activation and Teff proliferation (Figure 1)
A) Reduction of inflammatory secreting T cells by co-culture with anti TNFα CAR Tregs or anti IFNγ CAR Tregs; B) Proliferation suppression assay. anti TNFα CAR Tregs and anti-IFNγ CAR Tregs show enhanced suppression against stimulated Tresp cells compared with untransduced Tregs. N=5. *=p<0.05
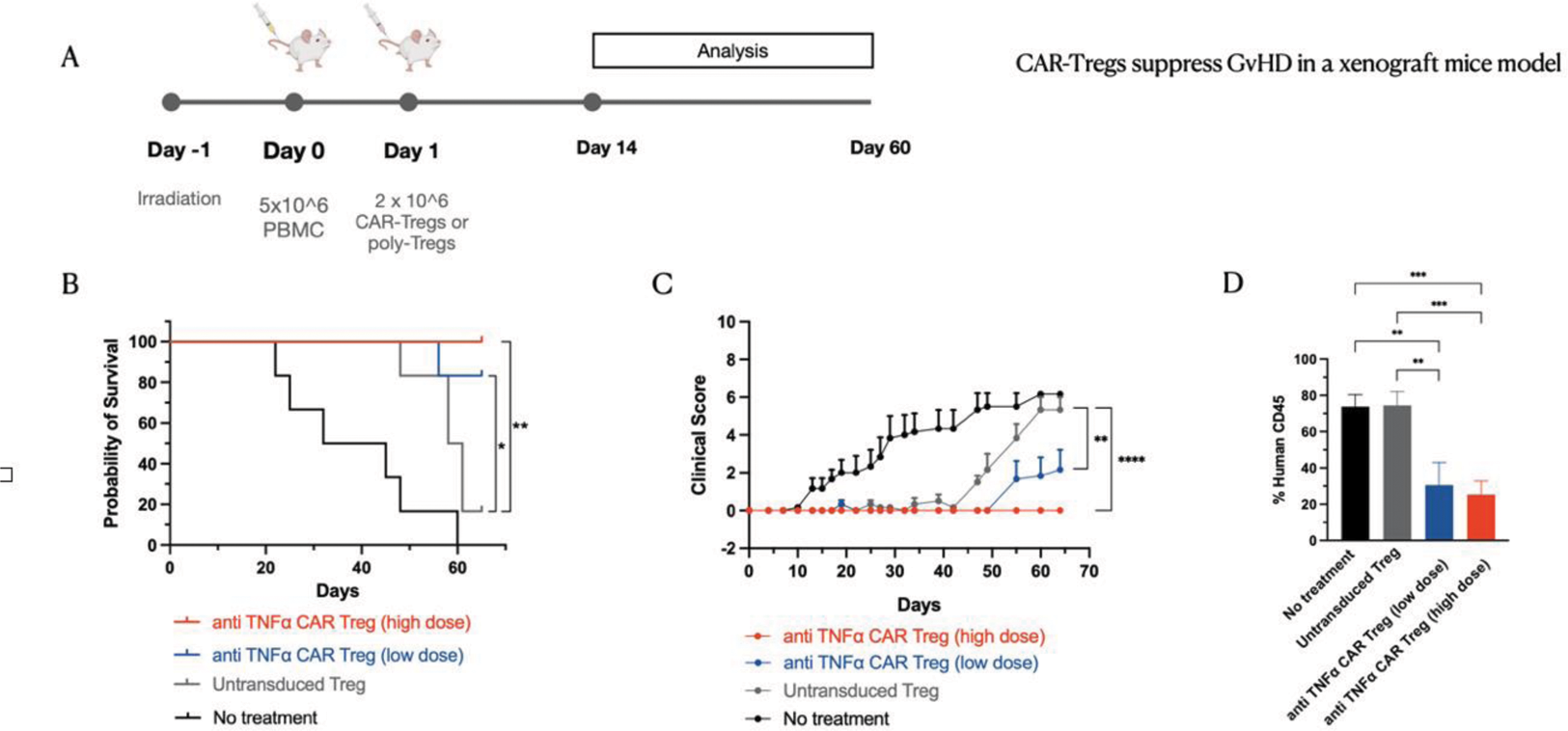
PanTreg are highly effective in controlling signs and outcomes of a humanised model of GVHD: We chose to test our TNF-specific scFv first in a humanised model of GFVD where TNF is relevant for the pathogenies. As shown in figure 2, below, we tested two different does of PanTreg and compared their efficacy against autologous polyclonal, untransduced Treg, with encouraging results in a dose-dependent fashion. We also show in figure 2D the effects of PanTreg on the proliferation in vivo of human effector T cells.
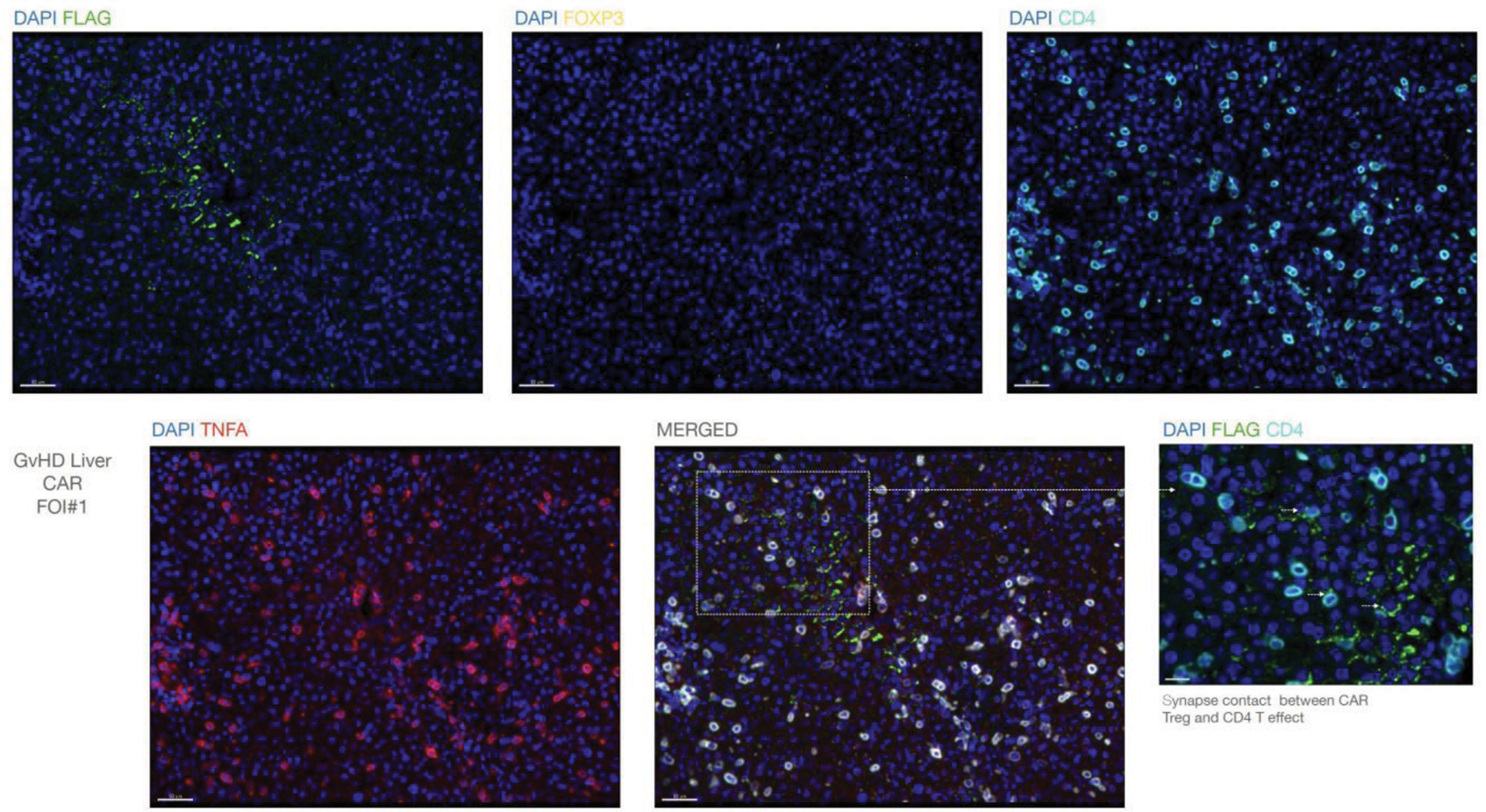
PanTreg home to inflammatory microenvironments, such as the liver in GVHD, and interact with CD4 cells producing TNF
Conclusion: We describe here our initial experience with a first in class type of CarTreg, where the scFv is specific for an inflammatory cytokine. This approach has the potential to provide with a single construct an innovative approach, and perhaps a permanent solution, to many diseases, in which such cytokines are relevant, by inducing active tolerance rather than suppressing, or “sponging out” the relevant cytokine.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.