

Background: Patients with immune-mediated rheumatic diseases treated with JAK inhibitors face an elevated risk of herpes zoster (HZ) infection. Shingrix, a recombinant inactive vaccine, offers protection against HZ. However, limited data exist on Shingrix vaccine responses in patients with immune-mediated rheumatic diseases (IMRD).
Objectives: This study aims to assess B-cell and T-cell immune responses elicited by herpes zoster vaccines in patients with rheumatic diseases undergoing treatment with JAK inhibitors (baricitinib, upadacitinib and tofacitinib), comparing these responses with those of healthy controls.
Methods: We investigated humoral, CD4, and CD8 immune responses in 43 JAK inhibitor-naïve patients with specific rheumatic diseases following a two-dose regimen of the Shingrix vaccine. The responses were compared with age, gender, and disease-matched healthy controls. Additionally, the serum cytokine profile (IL-17, IL-4, IL-6, IL-10, IL-2, TNF-α, INF- γ), including the expression of granzyme A and B and the level of VZV IgG antibodies post-vaccination, was assessed.
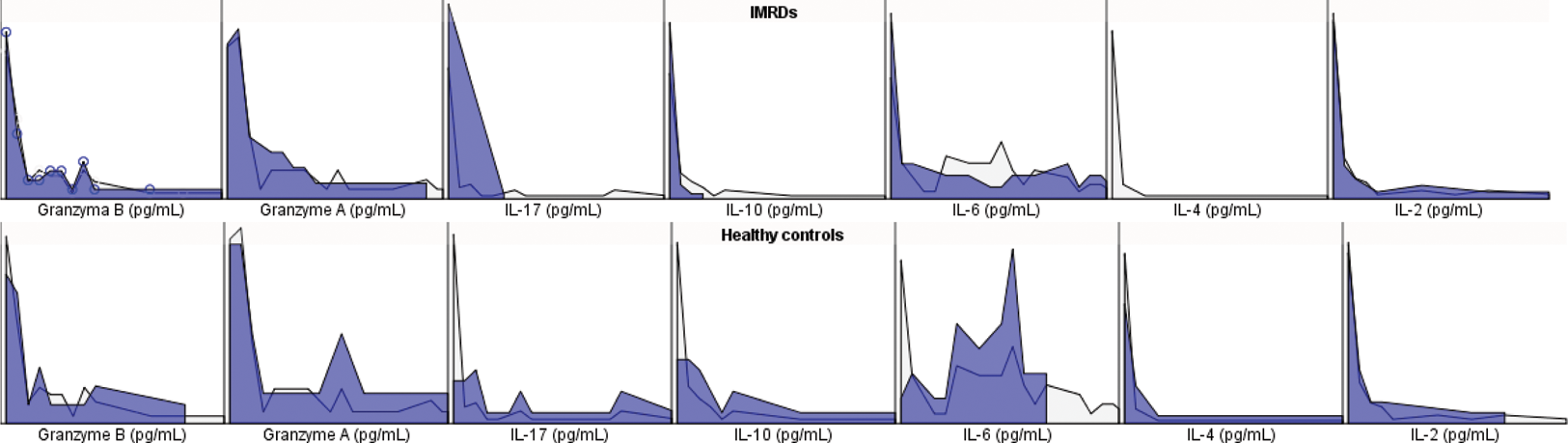
Results: Patients with IMRDs treated with JAK inhibitors exhibited lower seroconversion rates than healthy controls (74% vs. 96%, p=0.04). T-CD4 cellular immune responses displayed a significant difference (81% vs. 4%, p<0.0001), as did T-CD8 cellular immune responses (26% vs. 67%, p=0.009). Table 1 details the circulating levels of granzyme A and B, along with serum cytokines in IMRD and healthy controls. Post-vaccination, IMRD patients revealed reduced levels of IL-17, INF-γ, IL-10, IL-6, and IL-4 compared to healthy counterparts (Table 1 and Figure 1). Notably, no distinct differences in seroconversion, antibody titers, T-CD4 and T-CD8 cellular immune response emerged between distinct treatment subgroups. Negative correlations were observed between VHZ IgG Ab and T-CD4 cellular response with age >60 years, disease duration, MTX cumulative dose, GC cumulative dose, history of treatment with 2 or more b/tsDMARDs, and (Table 2). Additionally, a positive correlation between T-CD4 and T-CD8 cellular response was identified (β =0.36, p=0.003).
Conclusion: Patients with IMRD treated with JAK inhibitors exhibit lower seroconversion rates and distinct T-CD4 and CD8 cell responses compared to healthy controls. The serum cytokine profile analysis profile analysis provides nuanced insights. Negative correlations between VHZ IgG antibodies, CD4 T-cell and CD8 T-cell response and various clinical factors were found. While no significant differences were observed among JAK inhibitor subgroups, the study emphasizes the need for tailored vaccination strategies in IMRD patients, considering their treatment regimens and clinical characteristics. The findings contribute valuable information for optimizing vaccine efficacy in this vulnerable patient population.
REFERENCES: NIL.
| IMRD | Healthy controls | P value | |
|---|---|---|---|
| VHZ IgG Ab (Ab Index) | 3.1 (1.2-5) | 4.8 (1-5) | 0.03 |
| Granzyme A (pg/mL) | 439,33 + 573,31 | 678,61 + 1061,8 | 0.44 |
| Granzyme B (pg/mL) | 133,98 + 207 | 138,61 + 151 | 0.31 |
| IL-17 (pg/mL) | 0 | 6.97 + 9.28 | <0.00001 |
| INF-γ (pg/mL) | 2.91 (0-117,38) | 94.9 (0.11-1484) | <0.00001 |
| TNF (pg/mL) | 501,01 (0-12234) | 98,98 (0-1579) | 0.35 |
| IL-10 (pg/mL) | 3,31 (0-35,48) | 38,98 (0-234) | <0.0001 |
| IL-6 (pg/mL) | 458,29 (0-6450) | 10039,43 (1-34434) | <0.0001 |
| IL-4 (pg/mL) | 0 | 0.36 (0-3.02) | <0.0001 |
| IL-2 (pg/mL) | 26 (0-478) | 44 (1.1-351.80) | 0.51 |
Correlations between humoral and cellular response
| β coefficient (p value ) | Age >60 years | Disease duration | Cumulative glucocorticoid dose | Cumulative methotrexate dose | Treatment with 2 or more b/tsDMARDs |
|---|---|---|---|---|---|
| VHZ IgG Ab | β = -0.29, p=0.02 | β = -0.24, p=0.04 | β =-0.22, p=0.04 | β =- 0.42, p=0.0007 | β =-0.351, p=0.003 |
| CD4 T-cell response | β =-0.46, p 0.0007 | β = - 0.643, p=0.0007 | β = -0.32, p=0.007 | β = -0.46, p=0.001 | β =-0.20, p=0.001 |
| CD8 T-cell response | β =0.155, p=0.2
| β =-0.24, p=0.04 | β =-0.19, p=0.1 | β = -0.18, p=0.13 | β = -0.19, p=0.12 |
Serum cytokines profile in IMRD and healthy controls.
Acknowledgements: NIL.
Disclosure of Interests: None declared.