

Background: Autologous anti-CD19 CAR T cells are showing early clinical evidence of safety and efficacy for the treatment of numerous autoimmune diseases[1]. Allogeneic CAR T cells have the potential to further address drug supply for the substantial B-cell driven autoimmune disease population with advantages that include scalability, removal of the need for patient apheresis, and reduced treatment wait times[2]. Emerging data in autoimmune disease trials suggests long-term CAR T cell persistence is not required as patients have achieved long-term remissions (> 1 year) despite the reconstitution of the B-cell compartment within months[3]. This suggests persistence-associated limitations of allogeneic CAR T cells observed in oncology[4] may not apply to the same extent to autoimmune disease.
KYV-201 is an investigational allogeneic anti-CD19 CAR T-cell therapy for B-cell driven autoimmune diseases[2]. KYV-201 combines the same fully human anti-CD19 CAR construct as KYV-101, an autologous CAR T-cell therapy being studied in lupus nephritis, systemic sclerosis, myasthenia gravis, and multiple sclerosis, with a differentiated allogeneic platform developed by Intellia Therapeutics involving selected CRISPR/Cas9-mediated gene edits[2,5]. These edits intend to avoid GvHD by removing the endogenous TCR and provide immune evasion to prolong CAR T cell persistence by removing key cell surface proteins involved in host recognition of an allogeneic cell product.
Objectives: To generate preclinical data supporting advancement of KYV-201 to first-in-human clinical studies.
Methods: CAR-mediated and target-dependent activity of KYV-201 against both CD19 + target cell lines and primary B cells was demonstrated with in vitro cytotoxicity, cytokine production, and proliferation studies, as well as with an in vivo xenograft model. Functionality of the immune evasion edits was demonstrated by in vitro assays modeling host T and NK cell-mediated rejection mechanisms. Lastly, potential genotoxicity concerns of the product were assessed through chromosomal translocation, karyotyping, and cytokine independent growth assays.
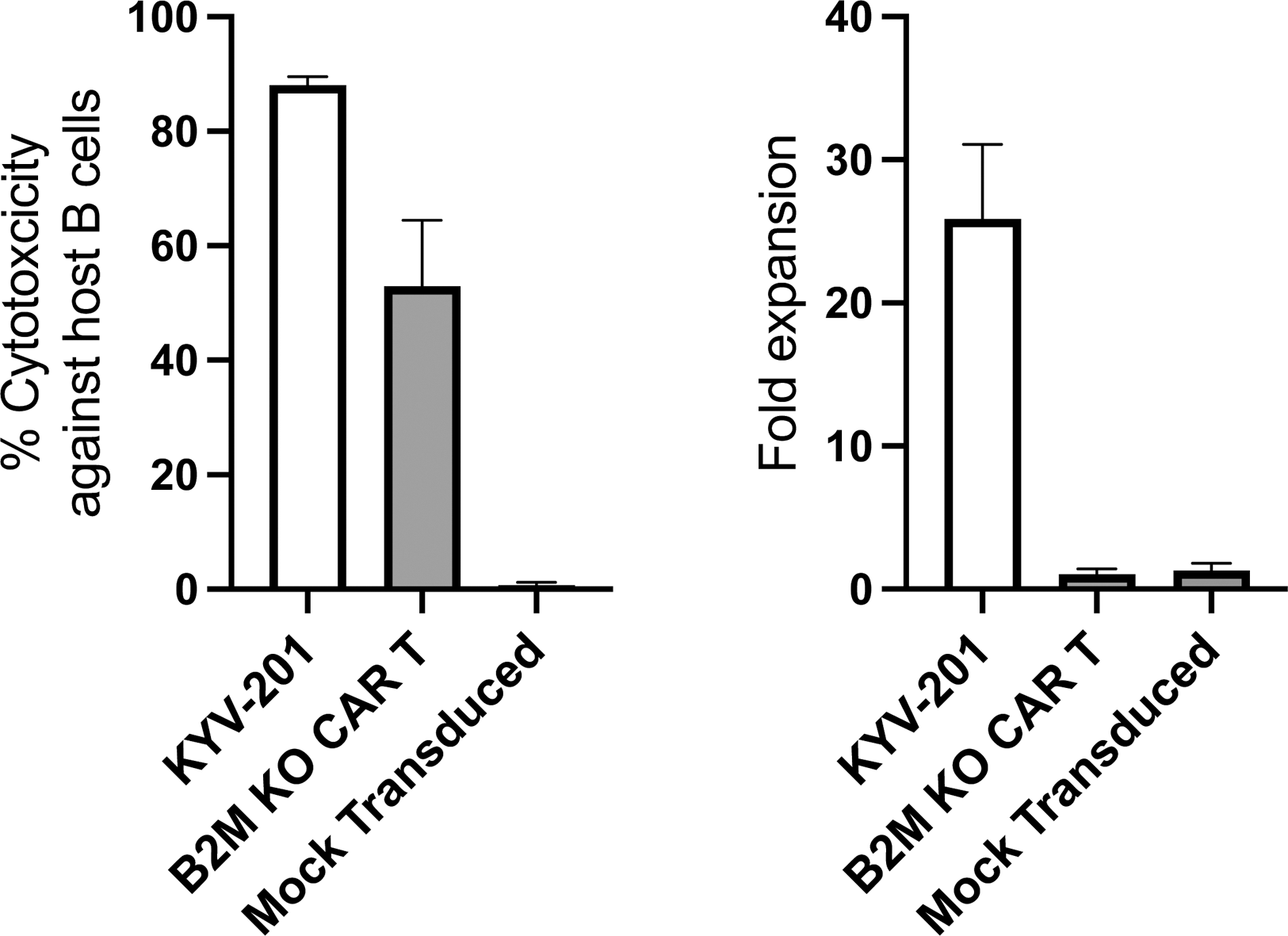
Results: KYV-201 demonstrated CAR-mediated and CD19-dependent cytotoxicity, cytokine production and proliferation against the human CD19 + NALM6 cell line. In vivo, KYV-201 effectively controlled growth of a CD19 + NALM6 xenograft in a dose-dependent manner. In co-culture with allogeneic T and NK cells, KYV-201 mitigated allogeneic recognition and rejection responses. In co-culture with allogeneic healthy donor PBMCs, KYV-201 eliminated primary B cells and proliferated in a CAR-dependent manner (Figure 1), avoiding rejection by alloreactive T cells and NK cells. Genotoxicity assays revealed minimal levels of translocations or chromosomal abnormalities by ddPCR and karyotyping, and a lack of transformation by cytokine independent growth assays.
Conclusion: The preclinical data generated for KYV-201 demonstrates the functionality and safety necessary to advance to first-in-human studies, bringing the promise of allogeneic CD19 CAR T cells for autoimmune diseases closer to fruition.
REFERENCES: [1] Schett G, et al. Lancet . 2023;402(10416):2034-2044.
[2] Blarcom TV, 8th Annual CAR TCR Summit; August 29, 2023; Boston, MA.
[3] Schett G, et al. ACR 2023, Abstract 0607. Arthritis Rheumatol . 2023;75(suppl 9).
[4] DiNofia AM, et al. Nat Rev Clin Oncol, 2021. 18:195–196.
[5] Taubmann J, et al. EULAR 2023, Abstract OP0141. Annals of the Rheumatic Diseases . 2023;82(1):93-94.
[6] Brudno JN et al. Nat Med , 2020. 26(2):270-280.
KYV-201, CAR T cells lacking HLA Class I (B2M KO CAR T cells), or mock transduced T cells were co-cultured with allogeneic PBMCs for 6 days at a (CAR) T cell:PBMC ratio of 1:30. KYV-201 demonstrated CAR-mediated cytotoxicity against primary B cells that was higher than that seen with B2M KO CAR T cells (left panel). KYV-201 expanded following CAR-mediated activation, in contrast to B2M KO CAR T cells that were mostly eliminated by the end of co-culture (right panel).
Acknowledgements: NIL.
Disclosure of Interests: Ashley Mahne Kyverna, Kyverna, Brandon Kwong Kyverna, Kyverna, Joseph Cheng Kyverna, Kyverna, Jessica Wang Kyverna, Kyverna, Jesus Banuelos Kyverna, Kyverna, Peter Starokadomskyy Kyverna, Kyverna, Soo Park Kyverna, Kyverna, Candice Gibson Kyverna, Kyverna, Shouvonik Sengupta Kyverna, Kyverna, Simone Sandoval Kyverna, Kyverna, Jazmin Bravo Kyverna, Kyverna, Jeanne Flandez Kyverna, Kyverna, Shairaz Shah Kyverna, Kyverna, Amanda Goodsell Kyverna, Kyverna, Yong Zhang Intellia Therapeutics, Ian Miller Intellia Therapeutics, Birgit Schultes Intellia Therapeutics, Tom Van Blarcom Kyverna, Kyverna.