

Background: Tumor necrosis factor inhibitors (TNFi) are generally the first class of biologic disease modifying anti-rheumatic drugs (DMARDs) prescribed for rheumatoid arthritis (RA) patients with an inadequate response to conventional synthetic DMARDs. Inadequate responses to TNFi range between 30% and 82%. 1–4 A molecular signal response classifier (MSRC) has been developed and previously validated to predict inadequate response to TNFi in RA patients and treatment selection informed by MSRC improves patient outcomes. 5,6
Objectives: Our study objective was to evaluate differences in first-line biologic treatment patterns for two groups of RA patients with moderate/high disease activity (CDAI>10), one group with MSRC testing and the other without. The MSRC-tested group was stratified according to MSRC results: one subgroup received a prediction of inadequate response to TNFi and the other received no signal of inadequate response.
Methods: An MSRC-tested cohort (n=301) of biologic naïve patients with CDAI scores>10 at the time of therapy initiation was drawn from the study to Accelerate Information of Molecular Signatures in RA (AIMS) and U.S.-based electronic health record (EHR) databases. All patients included started their first biologic treatment between 2021 and 2023. A second cohort of biologic naïve patients (n=2502) with CDAI>10 without MSRC results was selected from the EHR database, prior to initiating biologic therapy in 2021. Statistical significance between two categorical variables (MSRC tested vs not tested) and between allocated therapies by number of patients were evaluated using Pearson Chi-squared independence tests. A z-test was used for statistical evaluations of proportions of TNFi use between groups: MSRC-tested and MSRC-not tested groups; and between no signal of inadequate response detected and signal of inadequate response groups.
Results: TNFi were prescribed as first-line therapy in 70.1% (1754/2502) of patients who were not tested by MSRC, and in 48.5% (146/301) of MSRC tested patients. In the group of 301 MSRC-tested patients, 60.5% (182/ 301) received a signal of inadequate response and 39.5% (119/301) did not receive a signal of inadequate response (p <0.001). Among patients who received an MSRC signal of inadequate response, 33.0% (60/182) were prescribed TNFi, while 71.4% (85/119) of patients without an MSRC signal of inadequate response received TNFi therapy (p <0.001). While there was a decrease in the proportion of MSRC-tested patients receiving TNFi therapy, there was a concomitant increase in the proportions of patients receiving JAK, T-cell, and IL-6 inhibitor therapies (p <0.001), see Table 1.
Conclusion: We build upon clinical utility evidence previously described for the MSRC and investigate how precision medicine tests improve patient outcomes in rheumatology when the MSRC is available compared to current care practices. 5,6 In this study, we further observed that an MSRC signal of inadequate response to TNFi therapies was associated with decreased utilization of TNFi therapy and greater use of alternate MOA therapies with a downstream result of a significant shift in the overall TNFi utilization among RA patients. These data add to the growing evidence supporting the impact of MSRC on both practice patterns and patient outcomes, with ongoing studies aimed at further substantiating these findings.
REFERENCES: [1] Weinblatt, M. E. et al . NEJM 340 , 253–9 (1999).
[2] Plenge, R. M. & Bridges, S. L. Arthritis Rheum 63 , 590–593 (2011).
[3] Pappas, D. A. et al. Ann Rheum Dis 80 , 96–102 (2021).
[4] Curtis, J. R., Kremer, J. M., Reed, G., John, A. K. & Pappas, D. A. ACR Open Rheumatol 4 , 65–73 (2021).
[5] Jones, A. et al. Expert Rev Mol Diagn 21 , 1235–1243 (2021).
[6] Curtis, J. R. et al. Expert Rev Mol Diagn 22 , 1–10 (2022).
| Biologic Mechanism | Non –MSRC-tested | MSRC-tested |
|---|---|---|
| TNFi | 1754 (70.01%) | 146 (48.50%) |
| JAK | 424 (16.95%) | 70 (23.26%) |
| T-Cell | 218 (8.7%) | 57 (18.94%) |
| IL-6 | 102 (4.07%) | 25 (8.21%) |
| B-Cell | 0 | 3 (0.99%) |
| IL-1ri | 4 (0.15%) | 0 |
| TOTAL | 2502 | 301 |
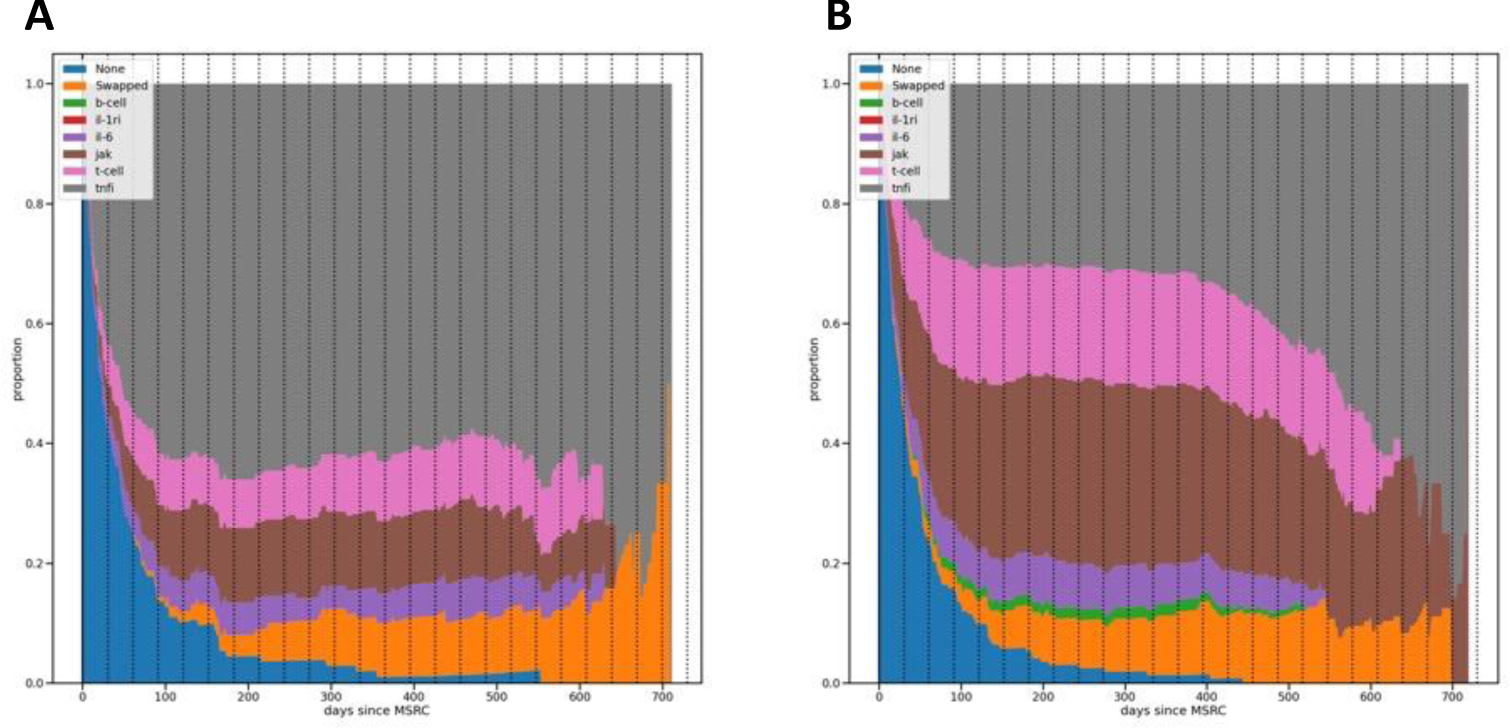
Proportions of therapies used in MSRC-tested patients, one group with no signal detected (A) and the other with a signal of inadequate response (B).
Acknowledgements: NIL.
Disclosure of Interests: George Karpouzas Janssen Pharmaceuticals, Scipher Medicine, Pfizer, Lixia Zhang Scipher Medicine, Mark C. Zielinski Scipher Medicine, Sherry Guardiano Scipher Medicine, Jennifer Dines Scipher Medicine.