

Background: Modic type 1 changes (MC1) are signal intensity changes on magnetic resonance imaging (MRI) associated with vertebrogenic pain. Endplate damage is associated with MC1, exposing immune-privileged disc tissue to the immune cells from adjacent bone marrow. Despite many studies pointing on the role of inflammation in MC1, a comprehensive investigation of immune system remodeling in MC1 lesions is missing, and that could reveal novel pathomechanisms associated with MC1.
Objectives: This study aimed to investigate changes in the bone marrow immune cell composition in MC1.
Methods: Vertebral bone marrow aspirates (n=22, MC1+intra-patient control= 8 + 8, control patients=6) from patients undergoing lumbar spinal fusion surgery were taken before screw insertion. Mononuclear cells from aspirates were isolated and cryopreserved. To screen changes in immune cell populations, we used cells from aspirates analyzed by a 36-color flow cytometry-based immunophenotyping panel combined with 360 PE-labeled antibodies against surface markers. To avoid batch effects, we optimized the multiplexing technique which allows the analysis of all samples in one tube. We utilized the presence of CD45 on the surface of all immune cells and stained it on each sample with different combinations of fluorescently labeled antibodies, to create a unique fluorescent barcode on each sample. Barcoded samples were multiplexed and co-stained with 36 antibodies against surface markers. This allowed us to distinguish B cells, T cells, NK cells, dendritic cells, monocytes/macrophages, basophils, and ILCs together with their activation and functional maturation status. Stained cells were divided into 360 aliquots, and each was co-stained with one additional PE-labeled antibody. Samples were analyzed on a spectral flow cytometer, computationally demultiplexed, and gated into immune cell populations, and the expression of screened markers on the surface of these populations was analyzed.
Results: Our analysis revealed significant changes in the lymphocyte subset proportions in the MC1 patient’s bone marrow. T cells, particularly NK-like T cells, and TCR Vδ1 + T cells, as well as IgG + and IgA + B cells, accumulated in MC1 patients compared to control (Figure 1A). Importantly, NK-like T cells and TCR Vδ1 + T cells were also significantly accumulating within the MRI lesions of MC1 patients compared to unaffected tissue from the same patient (Figure 1B). Accumulating T-cells in MC1 bone marrow exhibited phenotypic alterations indicative of changes in T cell functional maturation. More specifically, a profound increase in effector memory (TEM [CCR7 - CD45RA - ] and TEMRA [CCR7 - CD45RA + ]) in both MC1 patients compared to control patients (Figure 1A) and MC1 lesions compared to intrapatient control suggests an ongoing stimulation of T cells (Figure 1B, C). Investigation of changes in surface markers’ expression on T-cells revealed a significant increase in markers associated with proliferation (CD71) and activation (CD151) (Figure 1D). Interestingly, T-cells infiltrating MC1 lesions express significantly more of the NK receptors NKG2D and NKp80 (Figure 1D, red). The expression of NKG2D is among all T cell subsets the highest on the surface of TCR Vδ1 + T cells (Figure 1E), while NKp80 is the most common on the surface of NK-like T cells (Figure 1F). It suggests that recognition of stress-induced molecules in MC1 lesion might be the reason for the observed accumulation of these T cell subsets.
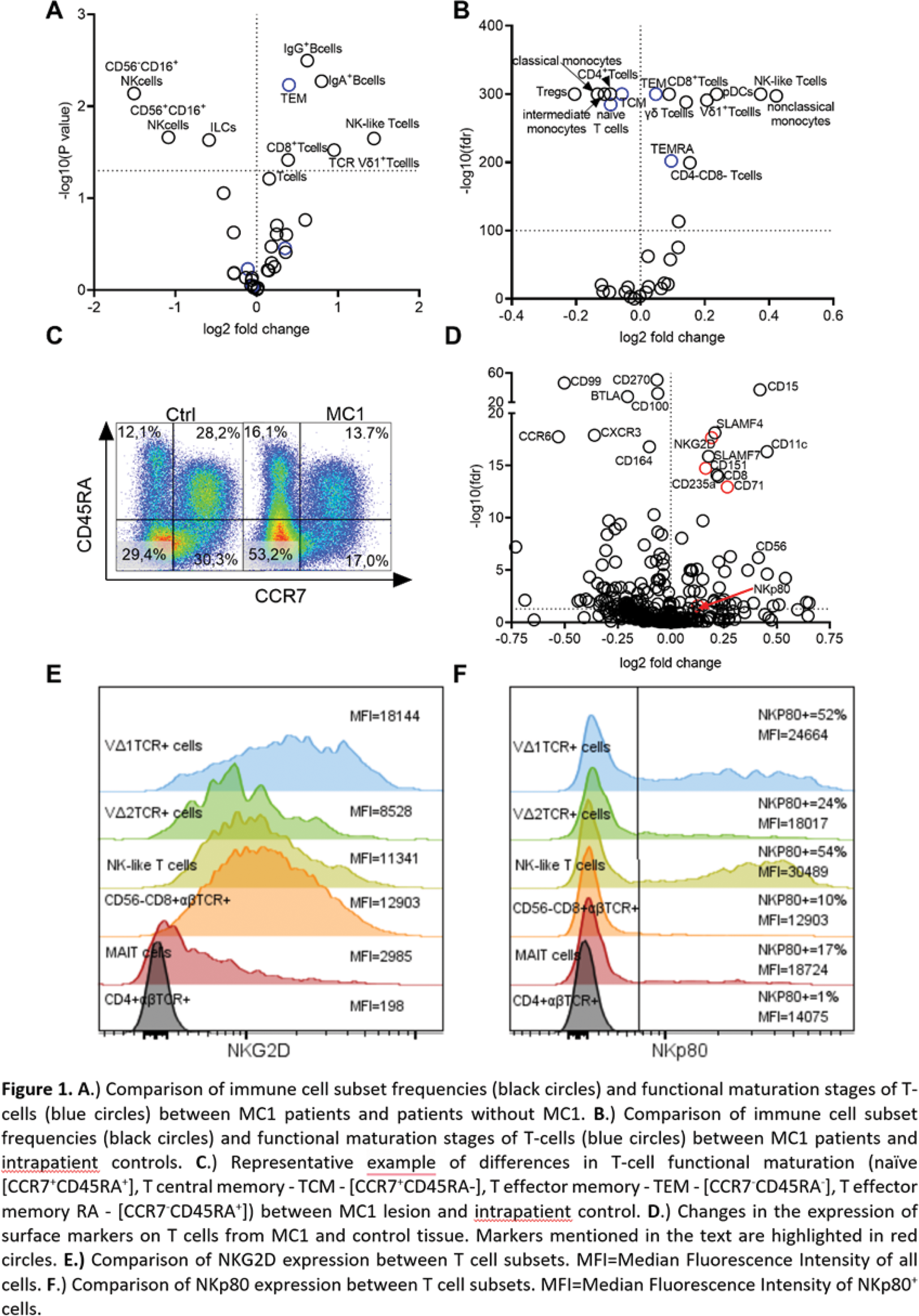
Conclusion: This ex vivo analysis provides the first direct evidence of specific T cell subset accumulations in MC1. We discovered an increased presence of NK-like T cells and TCR Vδ1 + T cells with the potential to react to stress-induced molecules. Future investigation of the role of these cells in MC1 might represent a key step toward understanding MC1 pathobiology and ultimately targeted treatments for MC1.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Jan Devan: None declared, Stefan Dudli: None declared, Nick Herger: None declared, Tamara Mengis: None declared, Irina Heggli: None declared, Melina Perez Vertti Valdes: None declared, Mazda Farshad: None declared, Oliver Distler CITUS AG, 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB, 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB.