

Background: Cutaneous lupus erythematosus (CLE) is a chronic inflammatory skin condition occurring alone or as a manifestation of systemic lupus erythematosus (SLE). With the lack of licensed treatments for CLE [1], there is an unmet need for new, safe, and effective treatments. Afimetoran is an investigational, first-in-class, orally bioavailable, potent, selective small molecule inhibitor of toll-like receptors (TLRs) 7 and 8. Afimetoran may have therapeutic potential for CLE, given the involvement of TLRs 7/8 in disease pathobiology of the closely related SLE [2]. A phase 1b, randomized, double-blind, placebo-controlled study (NCT04493541) demonstrated the preliminary safety, tolerability, and efficacy of afimetoran in patients (pts) with active CLE [3].
Objectives: To assess the pharmacodynamic effects of afimetoran, its impact on CLE pathobiology, and exploratory endpoints of clinical efficacy.
Methods: Pts were aged 18–65 years and had either SLE with cutaneous manifestations per EULAR/ACR 2019 classification criteria [4] or biopsy-proven CLE, with a modified CLE Disease Area and Severity Index-Activity (CLASI-A) score ≥ 6. Pts were randomized 2:1 to once-daily oral afimetoran (30 mg) or placebo (PBO) for 16 weeks with 4-week post-treatment follow-up. The primary endpoints were safety and tolerability; efficacy was an exploratory endpoint. Transcriptomics and cytokine analyses were performed using peripheral whole blood and serum, respectively, at each study visit, ie, baseline (pre-dosing), week (Wk) 1, Wk 2, Wk 4, Wk 8, Wk 12, and Wk 16 (during dosing) and Wk 20 (post-dosing). Separate statistical analyses were performed to pool and compare data from 13 patients and 13 age-, ethnicity-, and gender-matched normal healthy volunteers (NHVs). Treatment responses were evaluated longitudinally in each treatment arm. The dataset was analyzed using a linear regression model (limma package in R). Gene set variation analysis (GSVA) was performed for pathway enrichment.
Results: 13 pts were randomized (afimetoran, n = 8; PBO, n = 5); 12 pts completed 16 weeks of treatment and 1 discontinued afimetoran treatment due to COVID-19. Afimetoran demonstrated a favorable safety profile and was well tolerated vs PBO in pts with CLE, with no serious adverse events. Compared with PBO, afimetoran resulted in greater reduction of CLASI-A scores as early as Wk 4, which persisted through Wk 16 and up to Wk 20 (Figure 1). Improvement in the molecular disease profile, compared between patient and NHV samples at baseline, was rapid (Wk 1), sustained throughout the treatment period (Wk 16), and maintained up to 4 weeks post-treatment (data not shown). Expression of IFN pathway genes in whole blood was significantly reduced as early as Wk 1 compared to baseline (ΔGSVA enrichment score [ES] = 1.57, P < 0.0001) and the PBO group (ΔGSVA ES = 0.56, P < 0.01), and maintained for 16 weeks of treatment and at least 4 weeks post-treatment (data not shown). Expression of the drug target TLR7 and TLR8 pathway genes (Figure 2A, B) and key cytokines like IL-6, TNFα, IL-18, IFNγ, CCL3/MiP1α, and CCL4/MiP1β was greatly reduced with afimetoran treatment, demonstrating its potent and sustained pharmacodynamic effect. GSVA showed robust pharmacodynamic activity on immune cell populations including both immature and activated dendritic cells (Figure 2C, D), macrophages, and IFN and inflammatory pathway signatures.
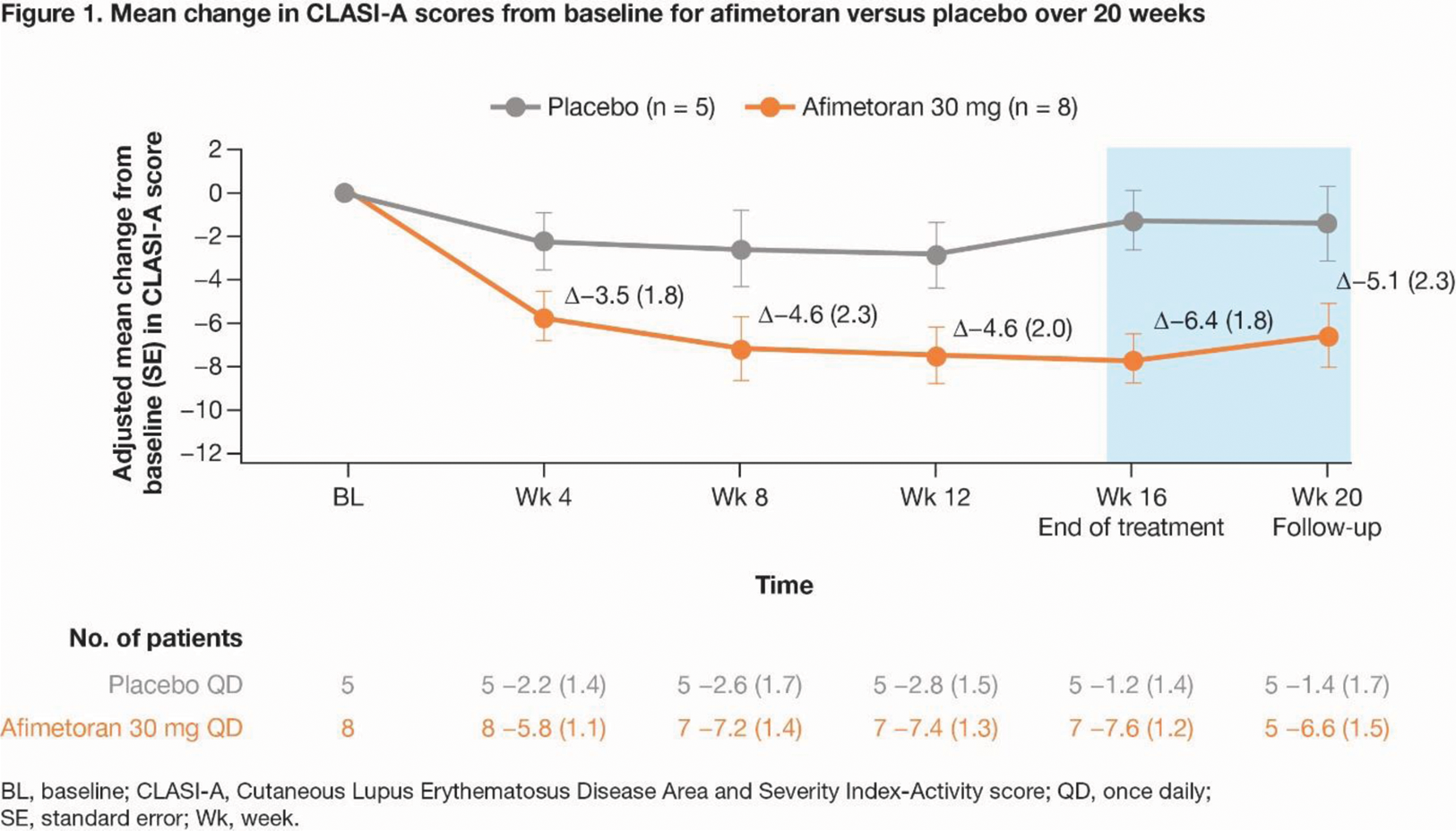
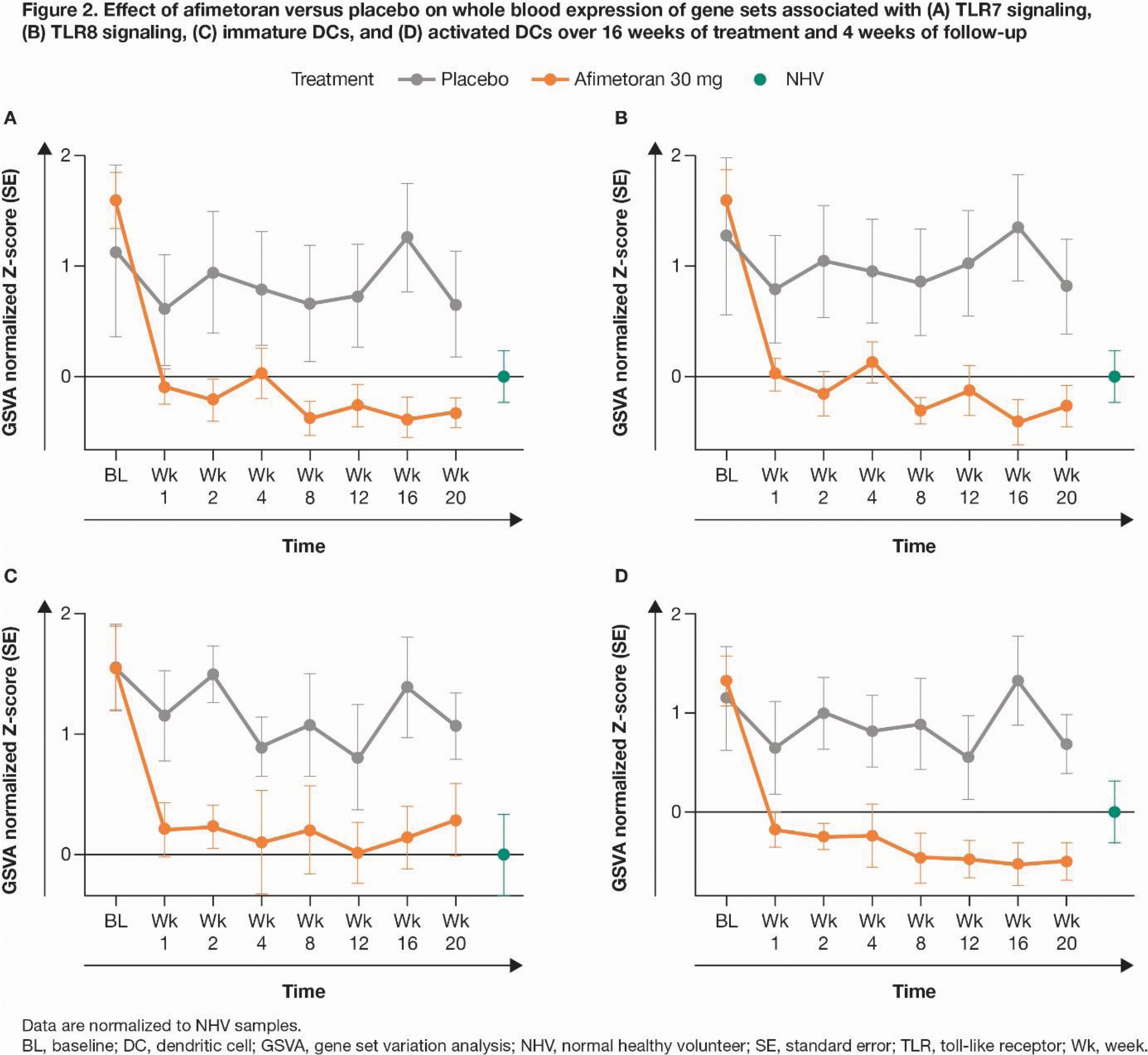
Conclusion: In pts with CLE, afimetoran was safe and well tolerated compared with PBO, and demonstrated treatment efficacy and potent pharmacodynamic effects early, throughout, and beyond the treatment period. The positive impact of afimetoran on the molecular disease profile and its potent pharmacodynamic and clinical effects suggest potentially substantial therapeutic benefit for patients with CLE.
REFERENCES: [1] Chang J, et al. Expert Rev Clin Immunol 2016;12:1109–1121.
[2] Ishizaka ST, et al. Eur J Pharmacol 2023;957:175962.
[3] Hosein F, et al. Arthritis Rheumatol 2023;75 (suppl 9):L17.
[4] Aringer M, et al. Arthritis Rheumatol 2019;71:1400–1412.
Acknowledgements: This study was funded by Bristol Myers Squibb. Professional medical writing and editorial assistance was provided by Candice Dcosta, MSc, of Caudex, a division of IPG Health Medical Communications, and was funded by Bristol Myers Squibb.
Disclosure of Interests: Frédéric Baribaud Bristol Myers Squibb, Janssen, Bristol Myers Squibb, Jasmine Saini Bristol Myers Squibb, Bristol Myers Squibb, Stanislav Ignatenko: None declared, Kristina Chadwick Bristol Myers Squibb, Bristol Myers Squibb, Lin Zhu Bristol Myers Squibb, Bristol Myers Squibb, Huynh Yen Thanh Bach Bristol Myers Squibb, Bristol Myers Squibb, Hazem Karabeber Bristol Myers Squibb, Bristol Myers Squibb, PRA Health sciences on assignment to Merck, Michelle Dawes Bristol Myers Squibb, Bristol Myers Squibb, Melanie Harrison Pfizer, Bristol Myers Squibb, Bristol Myers Squibb, Leon Carayannopoulos Bristol Myers Squibb, Bristol Myers Squibb, Gopal Krishna Bristol Myers Squibb, Bristol Myers Squibb, Fareeda Hosein Bristol Myers Squibb, Bristol Myers Squibb.