

Background: The adjuvanted recombinant glycoprotein E (gE) herpes zoster vaccine (RZV) received expanded approval for use in 2021 for adults receiving immunosuppression. Despite approval, little clinical or immunogenicity data exist of RZV in rheumatoid arthritis (RA) patients who are at high risk of herpes zoster (HZ). Further, it is unclear if biologics and other immunosuppressive therapies used in RA could diminish vaccine response. Accordingly, we evaluated the immunogenicity and safety of RZV in RA patients using the T-cell co-stimulatory inhibitor abatacept.
Objectives: To assess the immunogenicity of RZV in patients with RA receiving intravenous or subcutaneous abatacept.
Methods: 70 participants aged ≥ 18 years with RA taking abatacept were randomized 4:1 to receive RZV or placebo at entry and then at week 8. Blood collected at weeks 0, 8, 12, and 60 was used to measure gE-specific cell-mediated immunity (CMI) by IFNg & IL2 dual-color FLUOROSPOT and IgG antibodies by ELISA. We defined a CMI response as ≥ 2-fold increase in IFN-γ or IL-2 spot forming cells (SFC) at week 12 and humoral response as ≥2-fold increase in IgG titer at week ≥8. We assessed RA flare using the OMERACT RA flare questionnaire (RA-FQ), completed at baseline and once a week for the 4-weeks after each vaccine dose, in addition to the clinical disease activity index (CDAI) and disease activity score-28 (DAS28-ESR) that were completed at baseline, week 8, and week 12. Our current analysis is preliminary and restricted to participants who have completed the study to date.
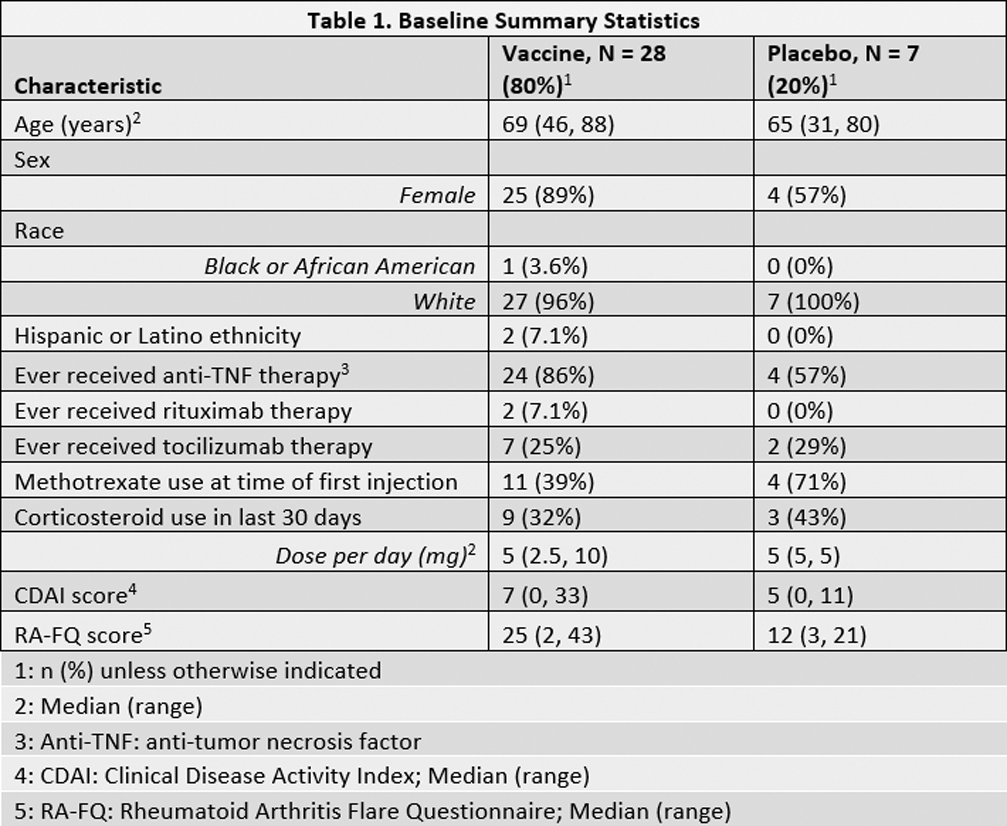
Results: The 35 (50%) participants who completed week 60 evaluations had median age 69 years (range, 31-88 years); 12 (34.3%) used corticosteroids within 30 days of week 0; and 15 (42.9%) were using methotrexate (MTX) at week 0 (Table 1). One participant in the placebo arm developed HZ between weeks 12 and 60 and was censored from the week 60 analyses. No other participants developed HZ during the study. All participants were included in the humoral immunity analysis, and 33 (94.3%) in the CMI analysis, with 2 excluded due to missing week 0 CMI measurements. At week 12, no participants in the vaccine arm achieved a ≥2-fold increase in either CMI marker. In contrast, 64.3% (95% CI 44.1, 81.4) developed ≥2-fold increase in anti-gE antibodies with geometric mean fold-rise (GMFR) of 2.72 (95% CI 2.1, 3.5) (Table 2). At week 60, only 21.4% (95% CI 8.30, 41.0) maintained a ≥2-fold antibody increase compared to week 0. In a subgroup analysis of the vaccine arm stratified by MTX use at week 0, CMI responses were nil for both groups and humoral responses were similar at week 12 (MTX use: 63.6% (95% CI 30.8, 89.1); no MTX use: 64.7% (95% CI 38.3, 85.8)). There were no responses in the placebo group. As defined by increase >12 in RA-FQ, >1.2 in DAS28, or >2 in CDAI from week 0 score, 12 (42.9%) participants in the vaccine arm and 3 (42.9%) in the placebo arm experienced new flares between week 0 and week 12. Among 28 participants that did not self-report a flare at week 0, 9 (40.9%) participants in the vaccine arm and 1 (16.7%) in the placebo arm self-reported a new flare between week 0 and week 12. Two serious adverse events were reported, and both judged unrelated to the vaccine.
Conclusion: In this ongoing study, people with RA who received RZV while using abatacept had poor vaccine responses, including a notable absence of CMI responses. Antibody responses to RZV were lower in magnitude and frequency than those previously described. These results raise the possibility that RZV may not confer adequate protection against HZ in people with RA taking abatacept. Future studies should assess whether holding abatacept around the time of vaccination would enhance vaccine immunogenicity. Clinical trial registered with

REFERENCES NIL. Acknowledgements: This study was supported through an Investigator-Initiated Research Grant from Bristol Myers Squibb. The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, United States, through Grant Award No. UL1TR002369. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Disclosure of Interests: Kevin L. Winthrop Pfizer, AbbVie, Union Chimique Belge (UCB), Eli Lilly & Company, Galapagos, GlaxoSmithKline (GSK), Roche, Gilead, BMS, Regeneron, Sanofi, AstraZeneca, Novartis, Moderna, BMS, Pfizer, Jeremy A. Hawkins: None declared, Adriana Weinberg GlaxoSmithKline, GlaxoSmithKline, Sarah A.R. Siegel: None declared, Jessica L. Baxter: None declared, Krystle Garth: None declared, J. Eugene Huffstutter: None declared, James Loveless: None declared, David Ridley: None declared, Elvia Moreta: None declared, Ilhem Messaoudi: None declared, Jeffrey R Curtis Abbvie, Amgen, Aqtual, Bendcare, BMS, FASTER, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, UCB, Abbvie, Amgen, Aqtual, Bendcare, BMS, CorEvitas, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, UCB