

Background: The ability of serum low-density lipoprotein to deliver cholesterol to cells is known as cholesterol loading capacity (CLC); in macrophages it associates with foam cell formation. In rheumatoid arthritis (RA), CLC was linked to long-term cardiovascular risk; it was also linked to coronary atherosclerosis burden and vulnerable plaque composition, particularly in biologic nonusers 1 . The relationship between changes in CLC over time and atherosclerosis progression is unknown.
Objectives: We here evaluated the influence of changes in cholesterol loading of macrophages on coronary plaque progression in patients with rheumatoid arthritis.
Methods: Atherosclerosis (noncalcified, partially or fully calcified plaques and coronary artery calcium [CAC] score) was evaluated with coronary computed tomography angiography in 140 patients without cardiovascular disease and reassessed in 100 after 6.9±0.4 years. Presence of 5 or more coronary plaques in a patient and lesions rendering greater than 50% luminal stenosis were considered extensive and obstructive disease respectively. CLC was measured as intracellular cholesterol content in serum treated human THP-1 monocyte-derived macrophages with a fluorimetric assay during baseline and follow-up atherosclerosis assessments. Multivariable negative binomial and robust linear regression tested the associations between changes in CLC from baseline to follow-up and coronary plaque and CAC progression and new extensive or obstructive disease respectively.
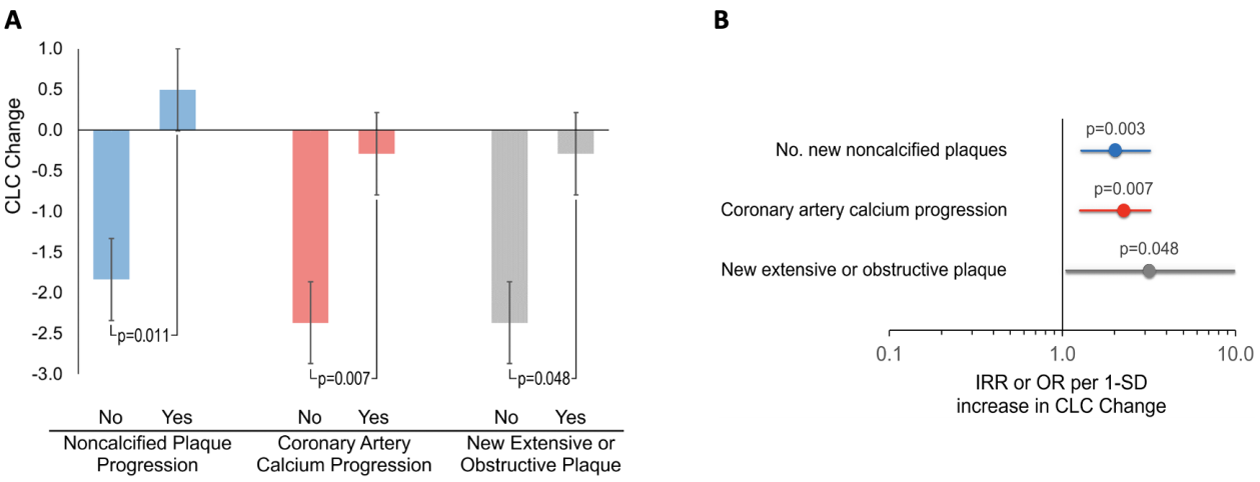
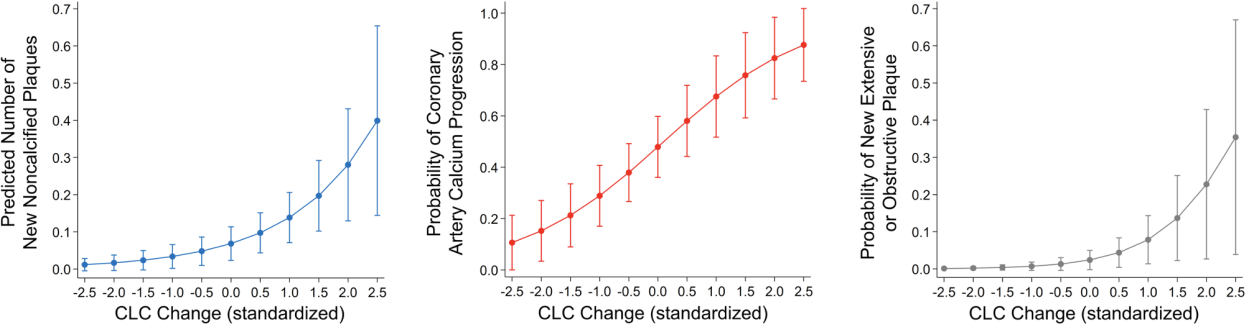
Results: CLC decreased in 68% and increased in 32% of patients at follow-up. All subjects (34/34) at the highest tertile of baseline CLC showed decrease at follow-up whereas 25/33 (75.8%) of patients at the lowest CLC tertile showed increase (p<0.0001). CLC change was higher in in patients with ≥1 new noncalcified plaques, CAC progression, or new extensive or obstructive disease compared to those without (Figure 1A). CLC change (per standard deviation) associated with higher number of new noncalcified plaques after adjusting for atherosclerotic cardiovascular disease (ASCVD) score, baseline plaque burden, obesity and duration of prednisone exposure throughout follow-up (incident rate ratio [IRR] 2.03, 95% confidence interval [95%CI] 1.27-3.24, p=0.003); greater likelihood of CAC increase after adjustments for ASCVD, baseline CAC, time-averaged c-reactive protein (TA-CRP), weighted daily-average atorvastatin equivalent dose and obesity (odds ratio [OR] 2.26, 95%CI 1.25-3.24, p=0.007) and higher risk of new extensive or obstructive disease at follow-up after adjusting for ASCVD, baseline plaque and TA-CRP (OR 3.17, 95%CI 1.01-9.94, p=0.048, Figure 1B). Incrementally higher CLC at follow-up compared to baseline associated with progressively more new noncalcified plaques, greater risk of CAC increase and new extensive or obstructive disease (Figure 2). In contrast, greater decreases in follow-up CLC associated with significantly fewer new noncalcified plaques, gradually lower risk of CAC increase and lower likelihood of new extensive or obstructive disease.
Conclusion: Increasing CLC and therefore rising cholesterol content in arterial wall macrophages over time associates with coronary atherosclerosis progression in a dose-dependent manner, and particularly higher burden of lipid rich noncalcified plaques and extensive or obstructive disease, all of which associate with greatest cardiovascular risk.
REFERENCES: [1] Karpouzas GA et al. RMD Open 2022;8:e002411
(A ) Differences in CLC change among patients with or without atherosclerosis progression (B ) Influence of CLC change on plaque progression.
Increasing CLC change associates with greater coronary atherosclerosis progression.
Acknowledgements: NIL.
Disclosure of Interests: George Karpouzas Janssen, Scipher, Pfizer, Bianca Papotti: None declared, Sarah Ormseth: None declared, Marcella Palumbo: None declared, Elizabeth Hernandez: None declared, Maria Pia Adorni: None declared, Francesca Zimetti: None declared, Matthew Budoff: None declared, Nicoletta Ronda: None declared.