

Background: Pathologic calcification of cartilage, characterized by the deposition of calcium-containing crystals, is a pivotal feature in osteoarthritis (OA). Lysyl oxidases (LOX(L)) are a group of enzymes catalyzing cross-link formation in collagen and elastin fibers. While their role in physiologic calcification of bone has been extensively elucidated, their involvement in pathologic cartilage calcification remains unexplored.
Objectives: To examine the involvement of LOX(L) in cartilage calcification, and the underlying mechanisms.
Methods: In vitro , murine and human chondrocytes were cultured in calcifying medium and treated with the pan-LOX(L) inhibitor β-aminoproprionitrile (BAPN). Human chondritic cell line hTC28.a was cultured in calcifying medium and treated with siLOX, siLOXL2 or siCtrl. Calcification was evaluated by Alizarin red; gene expression by qRT-PCR; fibrosis, hypertrophy, LOX and LOXL2 by immunohistochemistry; IL-6 secretion by ELISA; ROS production by fluorometric assay. In vivo , meniscectomized mice were treated with either PBS or BAPN over two months post-surgery. Knee calcific deposits were analyzed by MicroCT scanner. To explore potential correlations between LOX(L) genes and calcification-related genes, we conducted a GeneBridge bioinformatics analysis.
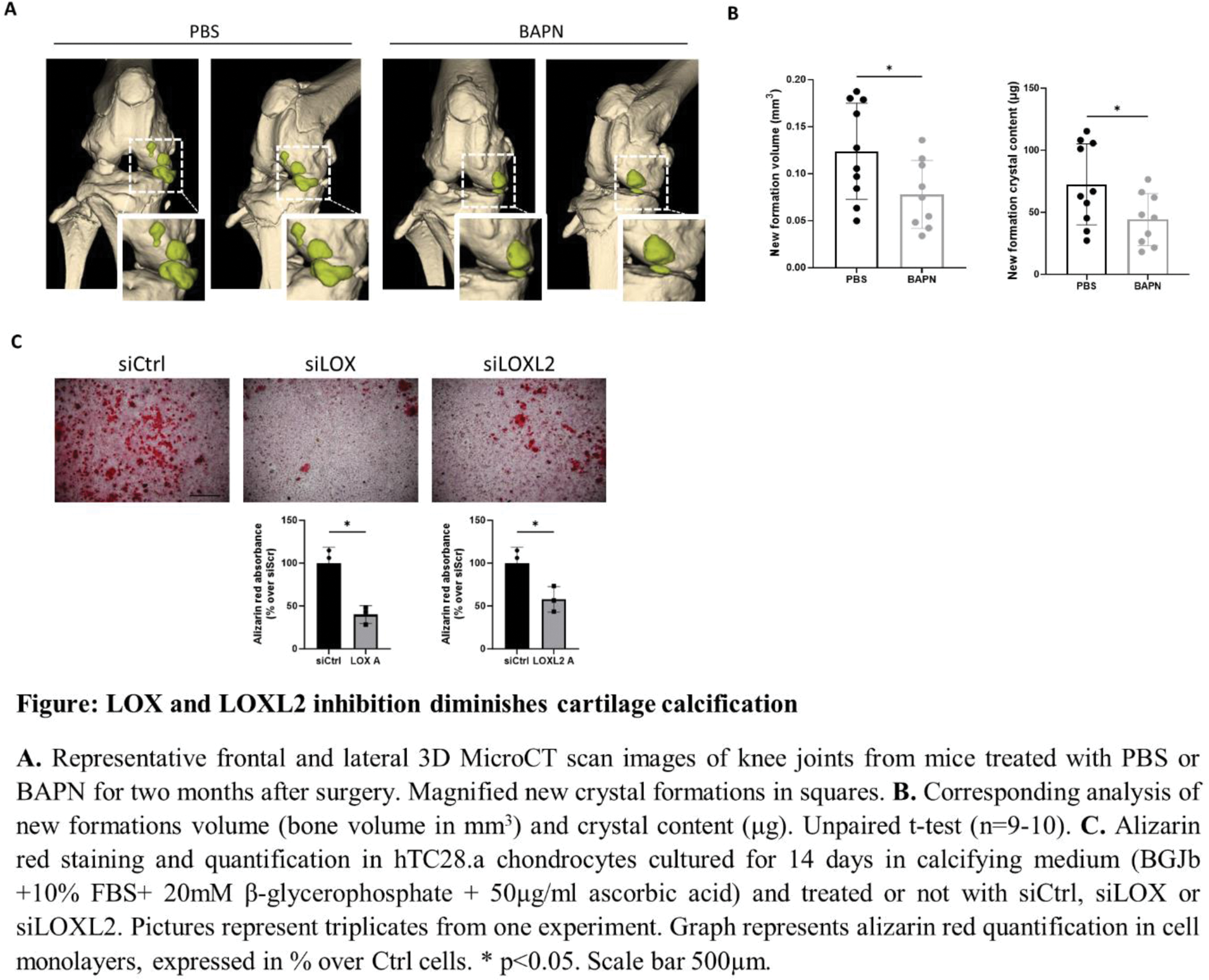
Results: LOX(L) inhibition by BAPN diminished crystal deposition in chondrocytes and in meniscectomized mice (Figure panels A and B). Underlying mechanisms included classical and non-classical roles of LOX(L). First, diminished cross-links in extracellular matrix suppressed chondrocyte calcification via downregulation of Anx5 , Enpp1 , Pit1 and Pit2 genes. Second, LOX(L) inhibition downregulated fibrotic markers Col1 and Col3 . Additionally, BAPN blocked chondrocytes hypertrophic differentiation, IL-6 release and ROS production, all triggers of cartilage calcification. Moreover, bioinformatics transcript analysis revealed that LOX(L)-containing module positively correlates with calcification, hypertrophy and catabolic enzymes modules. This association was conserved through species (mouse and human) and through different potentially calcifying tissues (kidney, arteries, skin). We specifically addressed which LOX was involved in these mechanisms and found LOX and LOX2 as the most expressed LOX(L) in cartilage. We found their upregulation in both the calcifying cartilage of meniscectomized murine OA and damaged human OA cartilage, compared to sham operated and undamaged cartilage respectively. Next, we performed in vitro LOX and LOXL2 RNA silencing (siLOX and siLOXL2 vs siCtrl). hTC28.a chondrocytes calcified less in the presence of siLOX and siLOXL2 in CM, as confirmed by alizarin red quantification (Figure panel C).
Conclusion: LOX and LOXL2 provide novel targets for future therapies aimed at treating pathologicmcartilage calcification in osteoarthritis.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.