

Background: Patients with X-linked agammaglobulinemia are susceptible to enterovirus (EV) infections [1]. Similarly, they have been described in patients treated with anti-CD20 monoclonal antibodies (mAbs) for hematological malignancies [2]. This risk has been poorly assessed in patients with autoimmune diseases (AIDs), in whom EV infections may be underdiagnosed due to non-specific clinical manifestations or AIDs mimicking organ involvement.
Objectives: We aimed to describe severe EV infections in patients receiving anti-CD20 mAbs for AIDs.
Methods: Patients were included following a screening of data collected by the French Enterovirus Surveillance Network (ESN) and a review of the literature. Only patients with a severe infection defined as a non-self-resolving infection with organ involvement were included.
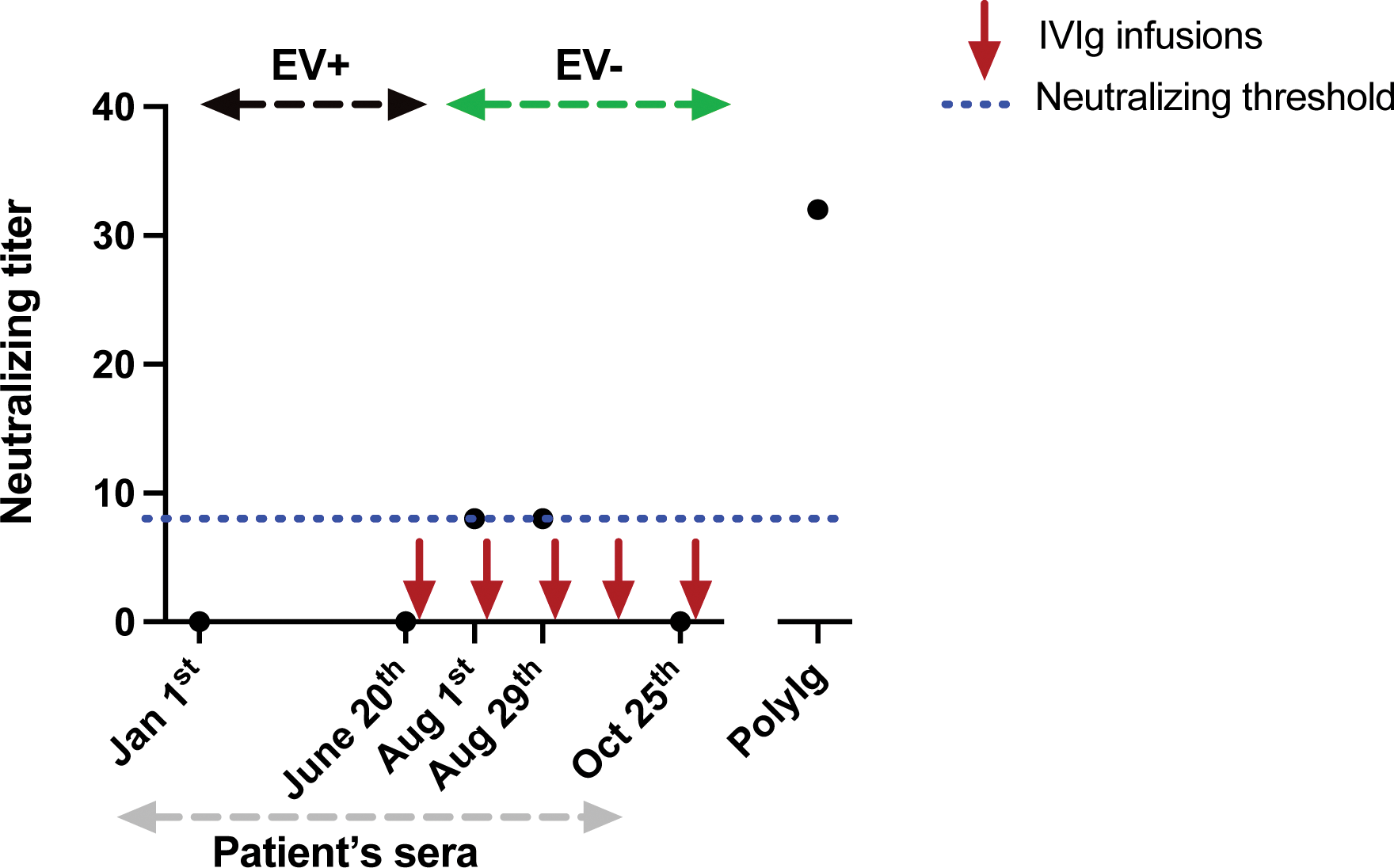
Results: Nine original cases and 17 previously published cases were included in the study (Table 1). Patients had received multiple anti-CD20 mAbs infusions (median 8 [5-10]), resulting in complete B-cell depletion and moderate hypogammaglobulinemia (median 4.9 g/L [4.3-6.7]), and had limited concomitant immunosuppressive treatments. Meningoencephalitis (n=21/26, 81%) associated with EV-positive cerebrospinal fluid (n=20/22, 91%) was the most common manifestation. The mortality rate was high (27%). EV was the only causal agents in all reported cases. Finally, in a patient with EV-A71 meningoencephalitis, a lack of B-cell response was shown (Figure 1).
Conclusion: EV genome detection should be part of the first intent microbiological screening in AIDs patients treated with anti-CD20 mAbs and presenting atypical and refractory organ involvement, especially meningoencephalitis, given the high mortality of this complication.
REFERENCES: [1] Halliday E. Enteroviral Infections in Primary Immunodeficiency (PID): A Survey of Morbidity and Mortality. Journal of Infection 2003; 46:1–8.
[2] Tellez R, Lastinger AM, Hogg JP. Chronic enteroviral meningoencephalitis in a patient on rituximab for the treatment of psoriatic arthritis: A case report and brief literature review. IDCases 2019; 17:e00558.
EV infection characteristics, management and outcome
| All cases
| Original cases
| Published cases
|
|
|---|---|---|---|
| Female | 19 (73%) | 6 (67%) | 13 (76%) |
| Autoimmune disease | |||
| Rheumatoid arthritis | 7 (27%) | 3 (33%) | 4 (24%) |
| Vasculitis | 4 (15%) | 2 (22%) | 2 (12%) |
| Primary autoimmune cytopenia | 4 (15%) | 0 | 4 (24%) |
| Multiple sclerosis | 3 (12%) | 2 (22%) | 1 (6%) |
| Systemic lupus | 2 (8%) | 1 (11%) | 1 (6%) |
| Minimal change disease | 2 (8%) | 0 | 2 (12%) |
| Others* | 4 (15%) | 1 (11%) | 3 (18%) |
| Biological characteristics | |||
| Gammaglobulin or IgG level (g/L), median [IQR] | 4.9 [4.3-6.7] | 5.0 [4.4-7.0] | 4.9 [4.2-6.0] |
| CD19+ B cells (/mm3), median [IQR] | 0 [0-0] | 0 [0-0] | 0 [0-0] |
| Immunosuppressive treatments | |||
| Lines of treatment before anti-CD20 mAbs, median [IQR] | 1 [0-3] | 1 [0-3] | 1 [1-3] |
| Last anti-CD20 mAbs infusion, months [IQR] | 6 [3-13] | 4 [3-7] | 8 [4-14] |
| Manifestations and organ involvement | |||
| Fever | 19 (73%) | 8 (89%) | 11 (65%) |
| Neurological | 21 (81%) | 8 (89%) | 13 (76%) |
| ENT | 12 (46%) | 4 (44%) | 8 (47%) |
| Muscular | 7 (27%) | 2 (22%) | 5 (29%) |
| Cardiac | 6 (23%) | 2 (22%) | 4 (24%) |
| Liver | 5 (19%) | 2 (22%) | 3 (18%) |
| Skin | 4 (15%) | 1 (11%) | 3 (18%) |
| Lung | 2 (8%) | 1 (11%) | 1 (6%) |
| Eye | 1 (4%) | 1 (11%) | 0 |
| Gut | 1 (4%) | 0 | 1 (6%) |
| Treatments | |||
| IVIg | 21 (81%) | 8 (89%) | 13 (76%) |
| Steroids | 6 (23%) | 2 (22%) | 4 (24%) |
| Organ transplant | 3 (12%) | 0 | 3 (18%) |
| Others | 6 (23%) | 0 | 6 (35%) |
| Outcomes | |||
| Survival without sequelae | 13 (50%) | 7 (78%) | 6 (35%) |
| Survival with sequelae | 6 (23%) | 1 (11%) | 5 (29%) |
| Death | 7 (27%) | 1 (11%) | 6 (35%) |
Legend : IQR: interquartile range, mAbs: monoclonal antibodies, MOG: myelin oligodendrocyte glycoprotein, d: day, TNF: tumor necrosis factor, ENT: ear-nose-throat, IVIg: intravenous immunoglobulin.
*Consisting in: thrombotic thrombocytopenic purpura (n=1), psoriatic arthritis (n=1), anti-MOG associated disease (n=1) and Devic’s disease (n=1).
Sera neutralization titer overtime in one of the reported patients with chronic EV-A71 meningoencephalitis
Legend : EV: enterovirus; EV+: positive EV RT-PCR in blood and CSF; EV-: negative EV RT-PCR in blood and CSF; PolyIg: polyvalent immunoglobulins used as a positive control. Each point represents a serum sample.
Acknowledgements: We thank for their help Dr Soumaya Sridi-Cheniti (Department of Radiology, CHU Bordeaux) and Dr Marie Jeanneau-Caron (Department of Pathology, CHU Bordeaux). We thank K. Coudéré and K. Benschop from the ENPEN network, who provided the polyIg sample and Jean-Luc Bailly (Auvergne University, LMGE UMR CNRS 6023, Team Epidemiology and pathophysiology of enterovirus Infection, Clermont-Ferrand, France) for his assistance with the serum neutralisation tests. We are grateful to all members of the EV surveillance network in Amiens (Dr Marie Louchet Ducoroy), Angers (Dr Caroline Lefeuvre, Pr Alexandra Ducancelle), Bayonne (Drs David Leyssene and Anne-Christine Jaouen), Besançon (Pr Quentin Lepiller), Bordeaux (Prs Marie-Edith Lafon and Sonia Burrel, Dr Camille Tumiotto), Bourgoin-Jallieu (Drs Tellini and Doat), Brest (Dr Léa Pilorgé and Pr Christopher Payan), Caen (Dr Cécile Schanen, Pr Astrid Vabret), Dijon (Dr Katia Balay, Pr Alexis de Rougemont), Frejus (Dr Gillon), Grenoble (Dr Sylvie Larrat), Lille (Dr Mouna Lazrek, Pr Didier Hober), Limoges (Prs Sylvie Rogez and Sophie Alain), Mantes-La-Jolie (Dr Emeline Riverain), Marseille (Drs Antoine Nougairède and Laetitia Ninove), Montpellier (Dr Vincent Foulongne, Pr Philippe Van de Perre), Nancy (Dr Véronique Vénard, Pr Evelyne Schvoerer), Nantes (Dr Marianne Coste-Burel), Nice (Dr Gonfrier), Orléans (Dr Clémence Guillaume and Jerôme Guinard), Paris – Cochin (Dr Anne-Sophie L’honneur, Pr Véronique Avettand Fenoël), Paris – Necker (Drs Hanène Abid, Marianne Burgard, Marianne Leruez-Ville), Paris – St Louis (Dr Maud Salmona, Pr Jérôme Legoff), Paris – Trousseau (Drs Kenda Saloum, Aurélie Schnuriger), Poitiers (Dr Agnès Beby-Defaux, Pr Nicolas Lévêque), Reims (Pr Laurent Andreoletti), Rennes (Dr Gisèle Lagathu, Pr Vincent Thibault), Roanne (Drs Jean-Benjamin Murat, C Brechet), Rouen (Dr Véronique Lémée, Pr Jean-Chirstophe Plantier), St Etienne (Dr Sylvie Pillet, Pr Bruno Pozzetto), Strasbourg (Dr Floriane Gallais, Pr Samira Fafi-Kremer), Suresnes (Dr Eric Farfour), Toulouse (Drs Jean-Michel Mansuy and Pauline Trémeaux, Pr Jacques Izopet), Toulon-CHI (Drs Anne-Lise Toyer and Cécile Poggi), Tours (Dr Karl Stefic, Pr Catherine Gaudy), Versailles (Dr Stéphanie Marque-Juillet), Villefranche (Dr Marine Jourdain).
Disclosure of Interests: None declared.