

Background: In recent decades, immune checkpoint inhibitors (ICIs) have gained popularity in the treatment of various cancers, but immune-related adverse events (irAEs) can impact the effectiveness of ICIs in killing tumors. Arthritis, occurring in approximately 10% of ICI-treated cancer patients, not only diminishes the quality of life but may also lead to prolonged inflammation [1]. Inbred lab mice are essential for researching and evaluating immune-based treatments for cancer and autoimmune diseases. However, a preclinical model for irAE arthritis has not yet been established. There is a need for treatment strategies that can control arthritis without compromising the efficacy of cancer immunotherapy. To address this, it is essential to establish an animal model inducing arthritis through the administration of immune checkpoint inhibitors in the presence of malignant tumors [2].
Objectives: The aim of this study is to establish a preclinical model for ICI-induced arthritis in the presence of tumors.
Methods: Collagen-Induced Arthritis (CIA) was selected as the arthritis model. Each mouse was transplanted with a tumor, followed by the administration of anti-PD-1 antibody and anti-CTLA4 antibody a few days later. Joint swelling alterations were examined to evaluate arthritis exacerbation by tumors/ICIs. After sacrifice, cytokine concentrations in the plasma were measured between groups. Additionally, gene expression in bulk joints was assessed using nCounter (Nanostring Technologies).
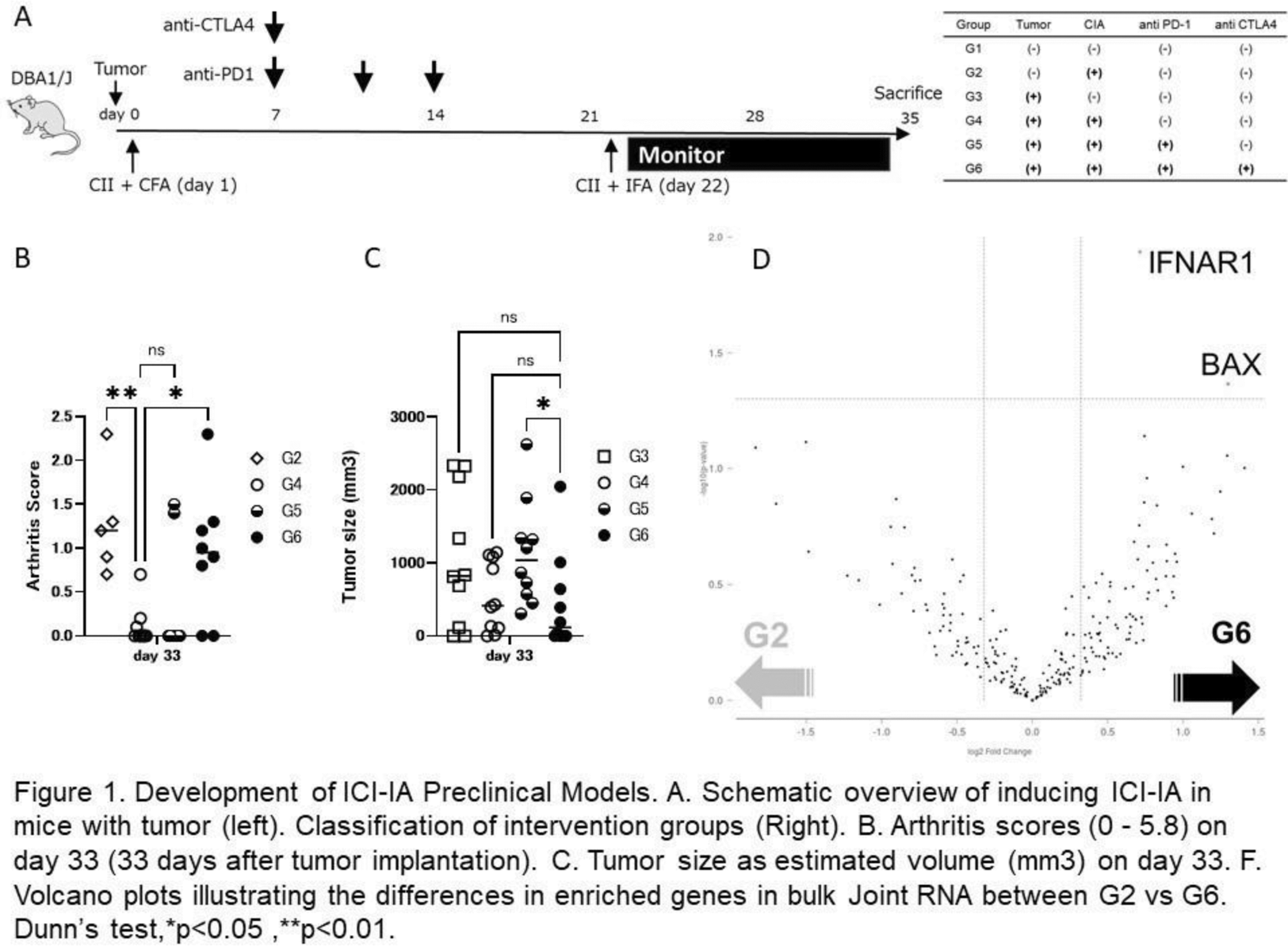
Results: Using the CIA model, the study protocol was stratified based on tumor presence, arthritis induction (utilizing a non-arthritis-induced model immunized with bovine serum albumin and adjuvant), and administration of ICI alone, anti-PD-1 antibody monotherapy, or combined therapy with anti-PD-1 and anti-CTLA4 antibodies (Figure 1). We observed a significant suppression of CIA induction by tumor transplantation, and this inhibitory effect was counteracted by combination ICI therapy, leading to arthritis exacerbation. Furthermore, tumor reduction effects were found only under CIA induction with combination ICI therapy. Gene analysis revealed a significant increase in IFNAR1 expression in the joint locality of Group 6 (with tumor/with ICIs) compared to Group 2 (without tumor/without ICIs), both of which induced arthritis equivalently. Plasma cytokine analysis showed that Group 6 had significantly lower IL-6 concentrations and significantly higher IFN-γ concentrations compared to Group 4 (with tumor/without ICIs).
Conclusion: CIA demonstrates a potent anti-arthritogenic effect in the presence of malignant tumors. The recurrence of arthritis with combination ICI therapy validates the CIA model as an appropriate animal experimental system for ICI-induced arthritis. This model has the potential to develop agents that improve arthritis without compromising therapeutic effects on malignant tumors and to investigate the pharmacological mechanisms underlying such effects.
REFERENCES: [1] Leipe J, Christ LA, Arnoldi AP, Mille E, Berger F, Heppt M, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open. 2018;4(2):e000714.
[2] Bayless NL, Bluestone JA, Bucktrout S, Butterfield LH, Jaffee EM, Koch CA, et al. Development of preclinical and clinical models for immune-related adverse events following checkpoint immunotherapy: a perspective from SITC and AACR. J Immunother Cancer. 2021;9(9).
Acknowledgements: NIL.
Disclosure of Interests: None declared.