

Background: We have previously shown that the matricellular protein CCN1, characterized by angiogenic and immunomodulatory properties, is overexpressed in endothelial cells and synovial tissue of patients with rheumatoid arthritis (RA).
Objectives: Our aim was to evaluate the effects of CCN1 inhibition in 2 complementary models of experimental arthritis.
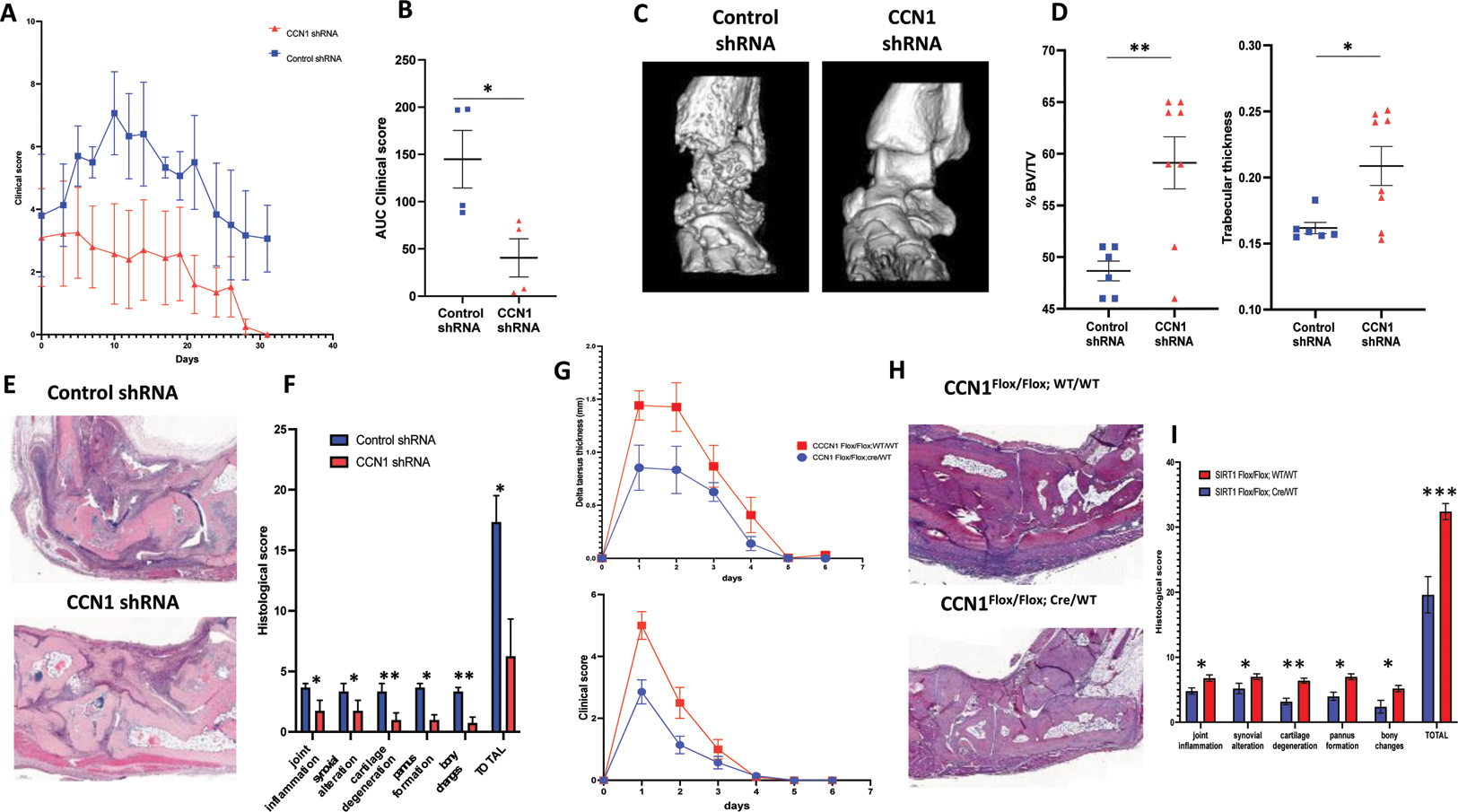
Methods: The effects of CCN1 inhibition achieved by local injection of a specific shRNA into the footpads were assessed in the TNF-α transgenic arthritis model (Tg197) (n=8 mice) by semi-quantitative clinical and histological scores. Structural damages were assessed in this model using microCT of the hind legs (tarsus and ankle). The consequences of conditional invalidation of CCN1 in endothelial cells (TEK-Cre-ERT2 mice; CCN1tm3.1fl) were analyzed in the murine model of methyl-BSA (mBSA)-induced arthritis (n=12 mice) by measuring tarsus thickness and semi-quantitative clinical and histological arthritis scores.
Results: We observed an improvement in the clinical score of CCN1 shRNA-treated Tg197 mice compared with control shRNA (Figure 1A). The AUC of the clinical score of CCN1 shRNA-injected mice decreased significantly by 73±5% (p=0.013) (Figure 1B). CCN1 shRNA-injected mice developed significantly less structural damage (Figure 1C). The % of bone volume to tissue volume (%BV/TV) was significantly higher by 10.5±3.0% in CCN1 shRNA-treated mice vs. control shRNA (p=0.005). Trabecular thickness (mm) was also significantly greater by 0.05±0.02 mm in mice treated with CCN1 shRNA vs. control shRNA (p=0.021) (Figure 1D). Histological analysis of hind paw sections after kerosene embedding confirmed previous results, with significantly less inflammation and structural damage in CCN1 shRNA-treated mice (Figure 1E and F).
Conditional inhibition of CCN1 in endothelial cells also significantly reduced the peak intensity of mBSA-induced arthritis observed on days 1 and 2 (Figure 1G). The area under the tarsal thickness curve and clinical score of CCN1-invalidated mice were reduced by 41±4% (p=0.032) and 58±7% (P=0.010). Histological analysis at day 6 showed a reduction in joint inflammation, synovial damage, cartilage degeneration, pannus formation and bone erosions in CCN1-invalidated mice (Figure 1H and I).
Conclusion: CCN1 inhibition significantly improved signs of TNF-α-induced experimental arthritis and prevented the development of structural damage in this model. The results obtained in the mBSA model illustrate the importance of endothelial CCN1 production in the development of arthritis. Reducing synovial angiogenesis can reduce structural damage in preclinical models of RA and could lead to the development of innovative therapies for RA, complementary to current treatments.
REFERENCES: NIL.
Effects of CCN1 inhibition and CCN1 endothelial invalidation on experimental arthritis. (A) Experimental arthritis in Tg197 mice receiving CCN1 or control shRNA locally into the left and right footpads. Y-axis shows clinical score. (B) area under the curve (AUC) of the clinical score (n=4 mice in each group). (C) Representative 3D reconstructions of ankles and tarsus of Tg197 mice treated with CCN1 or control shRNA. (D) Y-axis shows bone volume/tissue volume (BV/TV), trabecular thickness of right and left ankles and tarsus. (E) Sections of ankle and tarsus joints from Tg197 mice receiving CCN1 or control shRNA stained with hematoxylin (scale bar = 200μm). (F) Histomorphometric analysis of the area of synovitis and bone destruction. (G) mBSA-induced arthritis in littermates of CCN1 Flox/Flox; WT/WT mice (n=7) and CCN1 Flox/Flox; Cre/WT mice (n=5). Y-axis shows tarsus thickness and clinical score. (H) Sections of ankle and tarsus joints from CCN1Flox/Flox;WT/WT mice and CCN1Flox/Flox;Cre/WT mice stained with hematoxylin at day 6 of arthritis (scale bar = 100μm). (I) Histomorphometric analysis of the area of synovitis and bone destruction. All data are shown as the median ± 95%CI. * p<0.05, **p<0.01 determined by the Mann Whitney U test. Data are representative of a single experiment.
Acknowledgements: NIL.
Disclosure of Interests: None declared.