

Background: Rheumatoid factors (RFs) are polyclonal autoantibodies which bind to distinct sites on the fragment crystallizable (Fc) domain of immunoglobulin Gs (IgGs) [1]. They are detected in 70–90% of patients with rheumatoid arthritis (RA) predominantly as immunoglobulin M (IgM) isotype. Patients with RA and high RF levels (>200 IU/ml) have poorer prognosis, more severe progressive disease, greater joint and bone destruction and increased cardiovascular disease [2–4]. They can also display a decreased response to most tumor necrosis factor inhibitor (TNFi) biologics. However, a recent post-hoc analysis of the phase 4 EXXELERATE trial (NCT01500278), showed that patients wit RA and high RF levels maintained higher drug concentrations and had better clinical outcomes when treated with the PEGylated Fc-free TNFi, certolizumab pegol (CZP), compared with the Fc-containing TNFi, adalimumab (ADA) [5].
Objectives: To determine the molecular basis and reactivities of RFs to TNFi biologics and hence begin to explain why patients with RA patients and high RF levels respond differently to the various TNFi therapies.
Methods: Purified monoclonal antibody preparations based on published RF sequences and purified TNFi biologics provided the basis of the in vitro studies. RF IgMs (RF-AN, RF-61, RF-Yes8cT56K) [6-8] and TNFi biologic ADA were expressed in a proprietary Chinese hamster ovary cell line and purified by standard methods. CZP was expressed in E. coli , purified and PEGylated according to manufacturing guidelines [9]. Surface plasmon resonance (SPR) and ELISA binding assays were used to assess the binding of RF IgMs to TNFi biologics. Dynamic light scattering (DLS) was used to assess protein complex size when a fixed concentration of single or multiple RF IgMs were incubated with increasing amounts of TNFi biologics. X-ray crystallography data and structural modelling using published PDB structures (5WUV, 1ADQ) were used to illustrate the structure of CZP and the locations of RFs’ binding interactions with human IgG1 Fc.
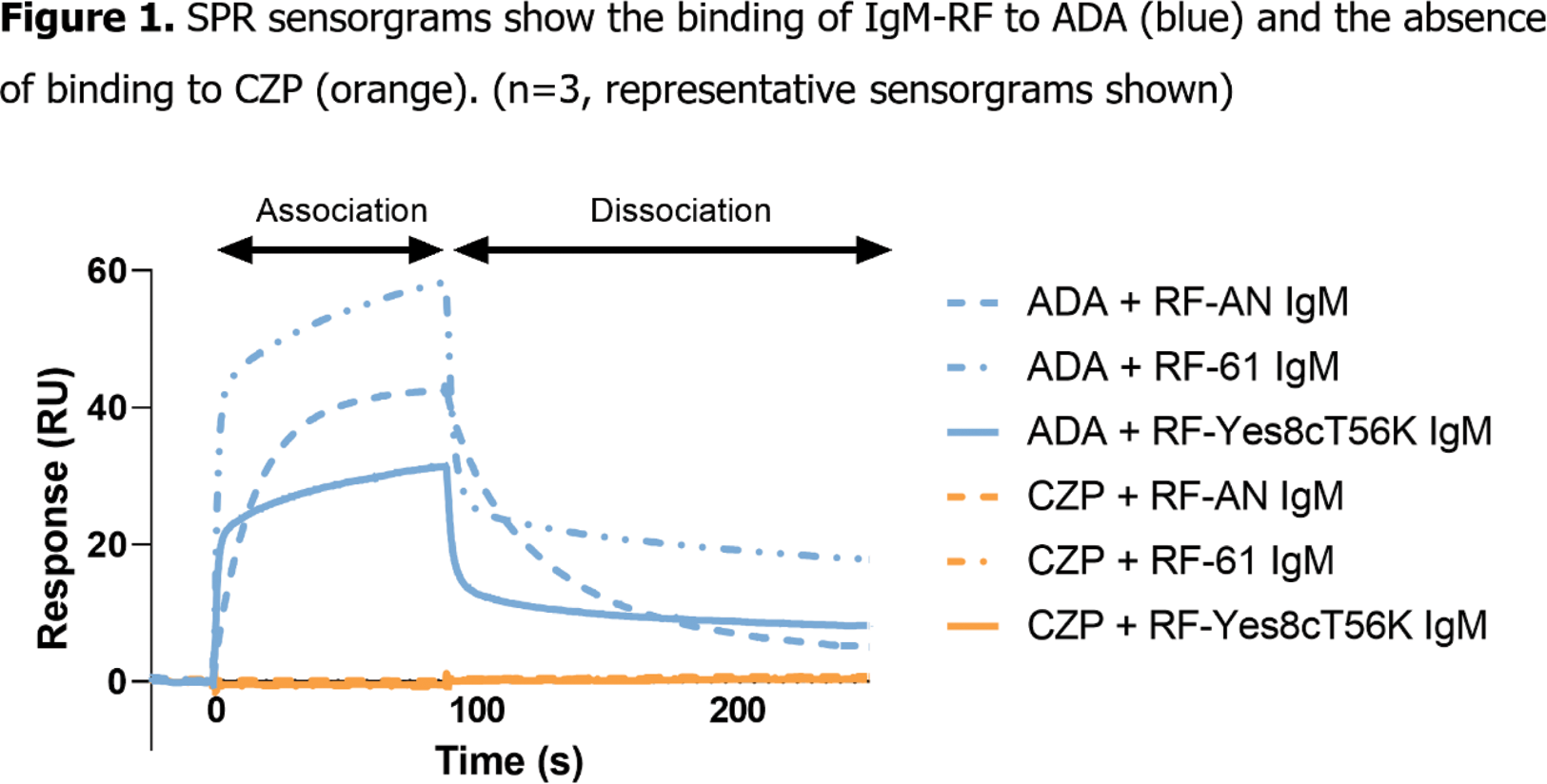
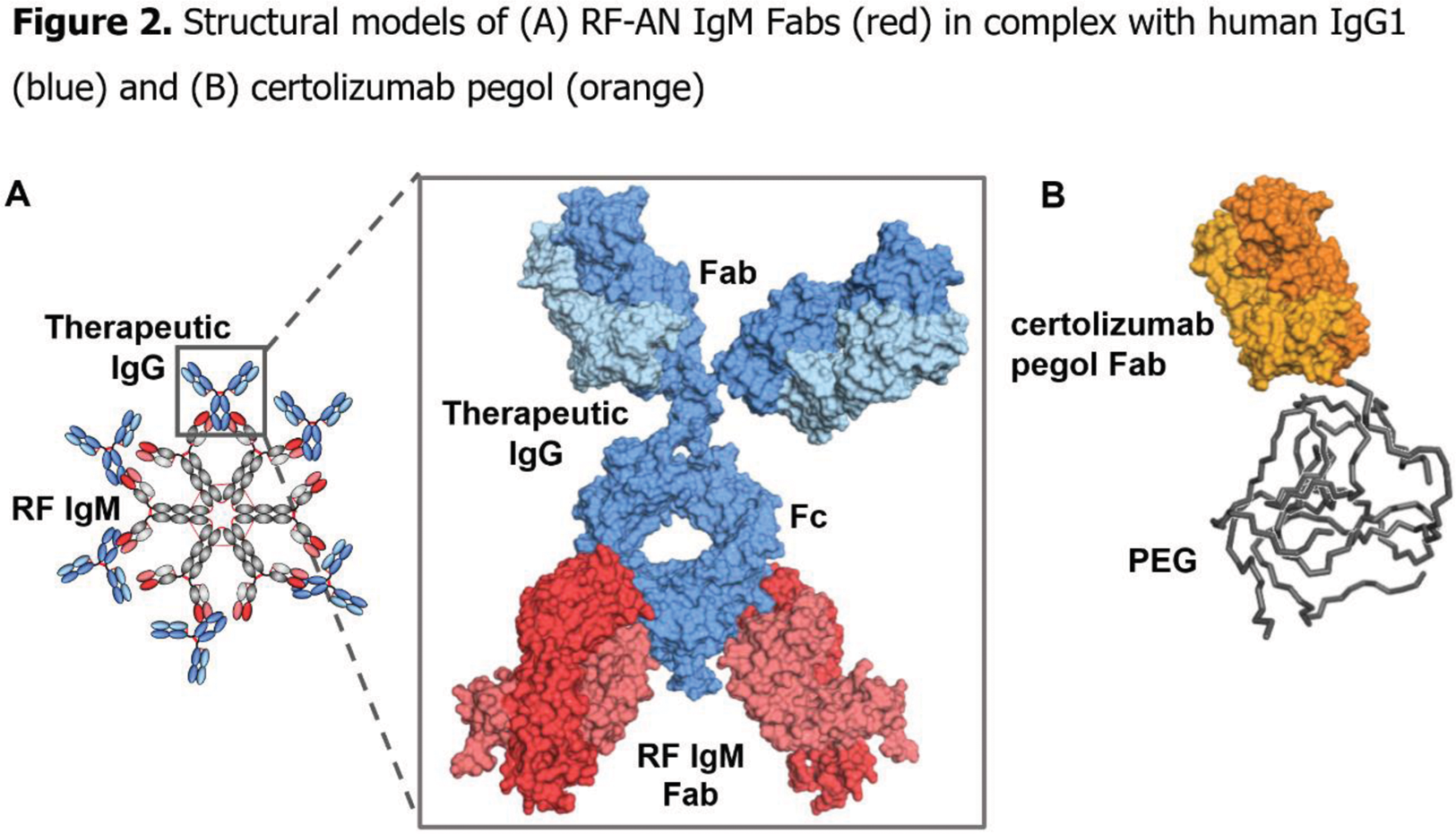
Results: ADA was bound by all three RF IgMs (RF-AN, RF-61, RF-Yes8cT56K) when assessed by both SPR and ELISA. No binding of CZP by any RF IgMs could be detected by either method (Figure 1). Existing X-ray co-structures show the fragment antigen binding (Fab) region of RF-AN IgM binding to human IgG1 Fc (Figure 2A). The crystal structure of CZP clarifies why RFs are unable to bind this TNFi, as CZP has no Fc domain (Figure 2B). When ADA was mixed with RF IgMs in solution, a new peak of greater hydrodynamic diameter was observed by DLS, indicating the formation of large complexes upon IgM RFs binding to ADA. When CZP and RF IgMs were co-incubated in solution, there was no increase in hydrodynamic diameter indicating that no complex was formed.
Conclusion: The RF IgMs all bound to Fc-containing ADA but were unable to interact with Fc-free CZP. The binding of ADA by the multivalent RF IgMs enabled formation of large protein complexes. These findings provide molecular insights into why patients with RA and high RF levels maintain higher drug concentrations and have better potential clinical outcomes when treated with CZP compared with ADA.
REFERENCES: [1] Gioud-Paquet M. Ann Rheum Dis. 1987;46(1):65–71.
[2] Albrecht K. Arthritis Res Ther. 2017;19(1):68.
[3] Sobhy N. Egypt Rheumatol. 2022;44(4):325–8.
[4] Fazeli MS. Clin Med Insights Arthritis Musculoskelet Disord. 2021;14:11795441211028751.
[5] Smolen J. ACR Convergence 2023;2148.
[6] Corper A. Nat Struct Biol 1997;4(5):374–81
[7] Duquerroy S. J Mol Biol. 2007;368(5):1321–31
[8] Shiroshi M. J Biol Chem. 2018;293(18):7008–16
[9] European Medicines Agency. Assessment Document for Cimzia. EMEA/664021/
Acknowledgements: Funded by UCB Biopharma
Disclosure of Interests: Susanna Bidgood UCB Pharma, UCB Pharma, David Kallenberg UCB Pharma, UCB Pharma, Alison Turner UCB Pharma, UCB Pharma, Jacqueline O’Neill UCB Pharma, UCB Pharma, Geofrey Odede UCB Pharma, Klaudia Mikula UCB Pharma, Jakub Zydron UCB Pharma, UCB Pharma, Hanna Hailu UCB Pharma, UCB Pharma, Ewa Lukijanczuk UCB Pharma, Sue Cross UCB Pharma, UCB Pharma, Kerry Tyson UCB Pharma, UCB Pharma, Sam Heywood UCB Pharma, UCB Pharma, Carlos Cara UCB Pharma, UCB Pharma, Bernard Lauwerys UCB Pharma, UCB Pharma, Baran Ufuktepe UCB Pharma, UCB Pharma, David Humphreys UCB Pharma, UCB Pharma, Anthony Shock UCB Pharma, UCB Pharma