

Background: Patients with lupus nephritis (LN) face 9x higher risk of premature cardiovascular disease (CVD) compared to patients with systemic lupus without nephritis and healthy peers. Renin-angiotensin system (RAS) blocking medications such as angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, are used to protect kidney function in patients with LN and proteinuria. The impact of RAS blocking therapy on CVD risk among LN patients is not well understood, and the limited studies evaluating this offer conflicting results. For example, a prior case-control study reported no cardioprotective effect of RAS blocking therapies in patients with LN [1], while another study reported fewer CVD events with RAS blocking therapy in patients with systemic lupus [2]. We sought to evaluate the impact of RAS blocking therapy on incident CVD events among patients with LN using our comprehensive, longitudinal LN clinical registry.
Objectives: Our aim was to examine the impact of RAS blocking therapy and treatment duration on CVD incidence over up to 18 years of follow up in a) all patients; and b) patients with no relative contraindications for RAS blocking therapies (such as glomerular filtration rate (eGFR) <30).
Methods: A clinical data registry was developed for adult LN patients who underwent an index kidney biopsy between 1999 and 2017 at a single academic center. Incident CVD-related deaths and non-fatal CVD events (ischemic heart disease, cerebrovascular accident, transient ischemic attack, and peripheral vascular disease) were adjudicated per guidelines. Prescription data were manually abstracted to calculate median treatment duration. Clinical and laboratory data at LN diagnosis were abstracted from electronic health records.
Associations between medications (RAS blocking therapy, aspirin, statin, chronic steroids, hydroxychloroquine, and other immunosuppression) and CVD events were examined using Cox Proportional Hazards Model in a) all LN patients; and b) patients with eGFR >30. Different RAS blockade exposure times (1, 5 years) were used in the model.
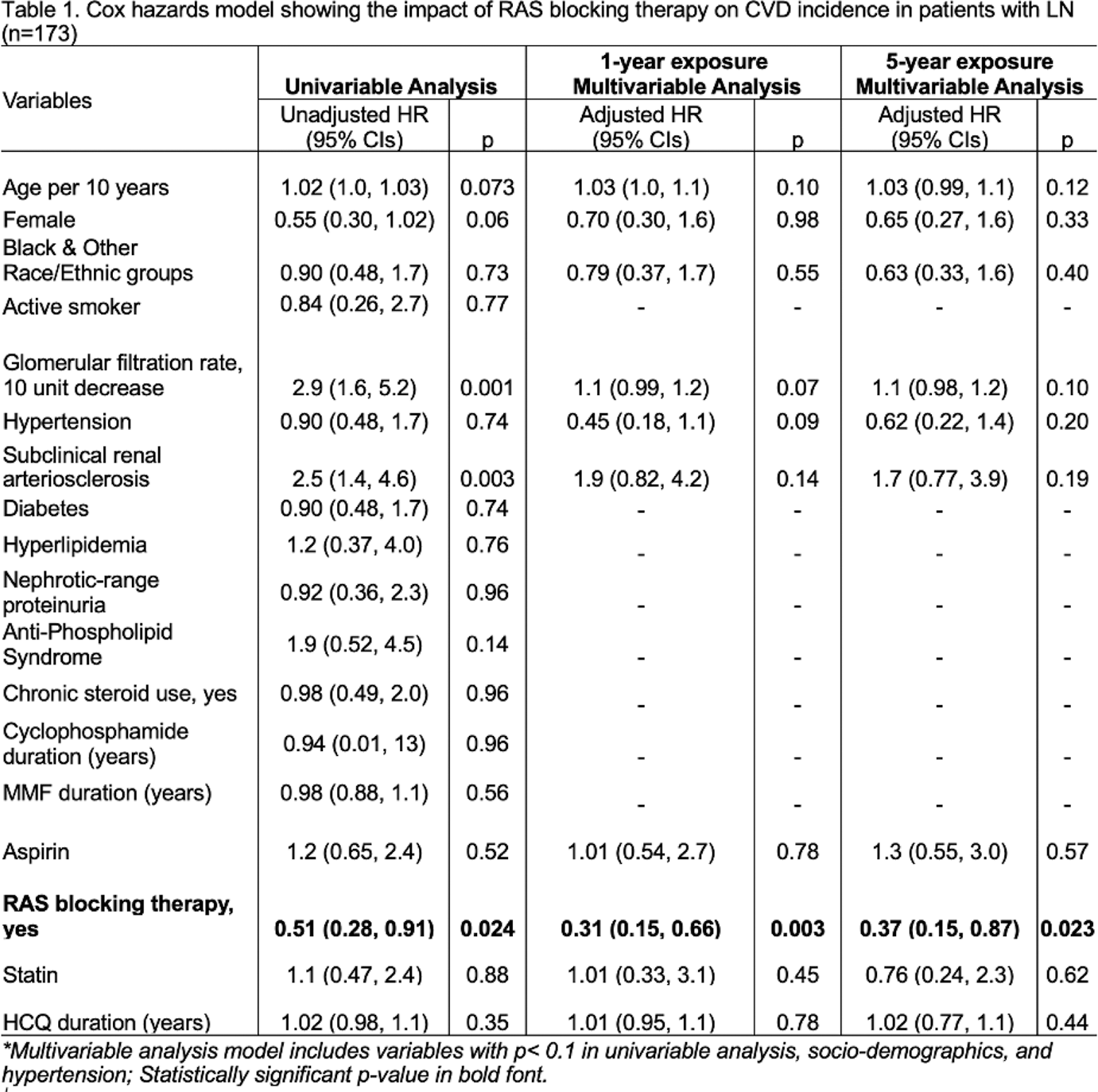
Results: Among 173 adult patients with incident LN, 40 incident CVD events including 14 CVD related deaths were observed one year after LN diagnosis until up to 18 years of follow-up. In all patients with LN, the use of RAS blocking therapy for at least one year after LN diagnosis reduced CVD occurrence by 69% (Adjusted HR 0.31, 95% CIs 0.15-0.66; Table 1). Likewise, prolonged use of RAS blocking therapy for 5 years was associated with 63% lower CVD occurrence after 5 years of LN diagnosis (Table 1).
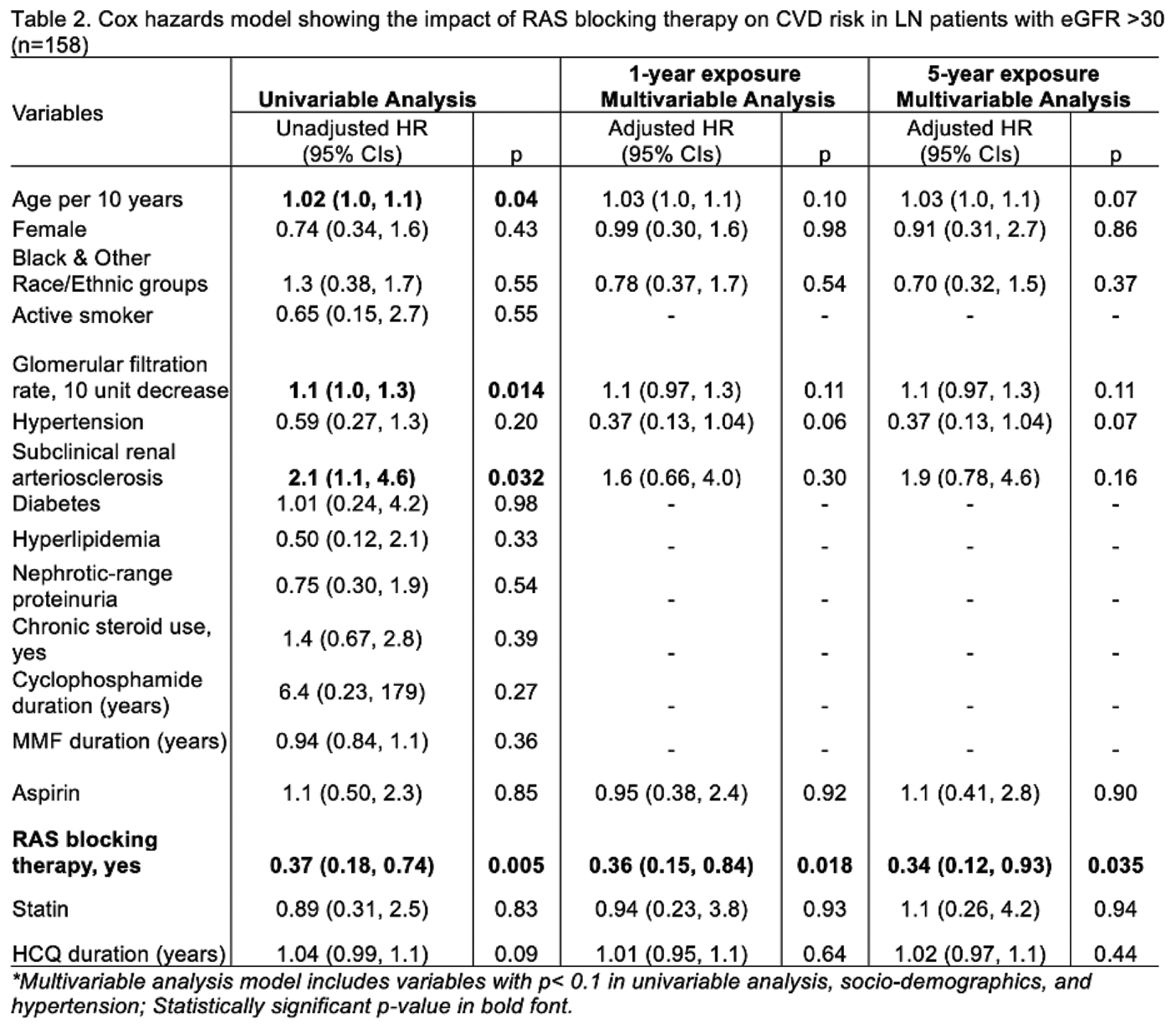
Among patients with eGFR >30 (n=158), RAS blocking therapy use for 1 year after LN diagnosis was associated with 64% fewer CVD events (Adjusted HR 0.36, 95% CIs 0.18-0.94, P=0.035; Table 2), and this association was maintained at 5 years of use (Table 2). Other therapies (e.g., steroids, aspirin, statin) were not associated with CVD risk (Tables 1 and 2). RAS blocking therapy was overlooked in 39% of patients with any CVD risk factors; among these, 45% developed a CVD event over time. The number needed to treat with RAS blocking therapy to prevent one additional CVD event was 6.
Conclusion: Early and prolonged use of RAS blocking therapy was associated with lower CVD incidence in LN. Using RAS blocking therapy in 6 additional patients with LN at diagnosis can prevent one additional CVD event. Cardioprotective effects of RAS blocking therapy should be considered during clinical decision-making, beyond known renoprotective effects, to improve health outcomes and survival in LN.
REFERENCES: [1] Tselios K et al. Does Renin-Angiotensin System Blockade Protect Lupus Nephritis Patients From Atherosclerotic Cardiovascular Events? A Case-Control Study. Arthritis Care Res. 2016.
[2] Hurst C et al. Renin-Angiotensin System-Modifying Antihypertensive Drugs Can Reduce the Risk of Cardiovascular Complications in Lupus: A Retrospective Cohort Study. Am J Med. 2023
Acknowledgements: The UW LN cohort is supported by the National Institutes of Health through a Clinical and Translational Science Award to UW ICTR.
Disclosure of Interests: None declared.