

Background: SARS-CoV-2 infections can cause severe inflammation and serve as a trigger for immune-mediated manifestations. Several case reports in adults have described auto-antibodies production (45-57%) and musculoskeletal (MSK) manifestations after the infection (2.7-5.9%), but in children the information is scarce [1-6].
Objectives: To describe the MSK manifestations, after SARS-CoV-2 infection, in a multicentric pediatric population without previously known rheumatic disease.
Methods: The clinical records of all new patients, between April 2020 to March 2023, from five centers, with MSK manifestations and/or serologic findings related to rheumatic diseases (RD) after SARS-CoV-2 infection, were reviewed. Based on available data in adults, 60 days were defined as the longest period of time between exposure to the virus and the onset of MSK manifestations or serologic findings. Patients with previous known RD were excluded. Data on demographic variables and clinical features were collected and presented as frequencies and median [interquartile range] for categorical and continuous variables, respectively.
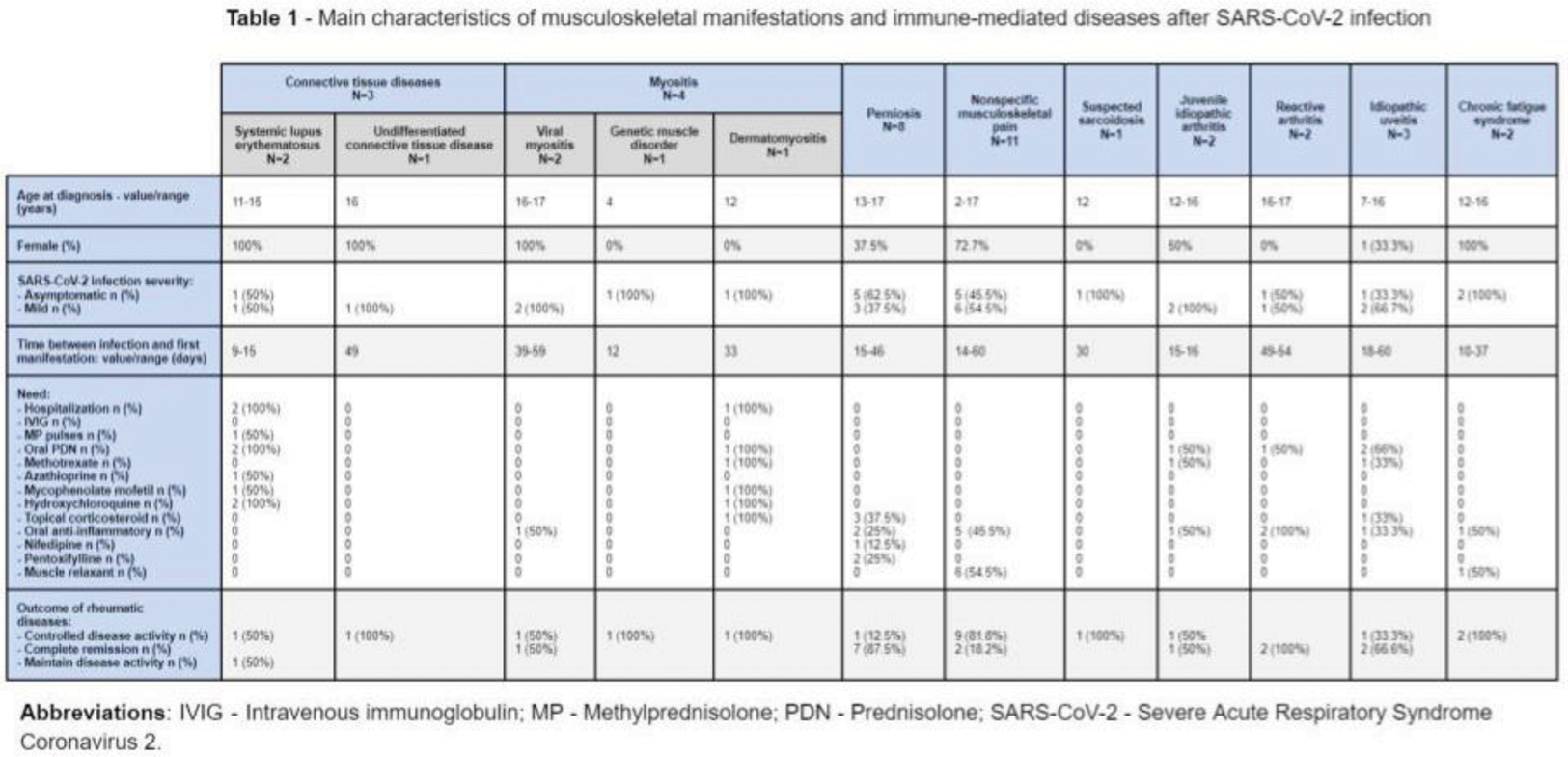
Results: During the study period 1063 new patients were observed, of which 36 (3.4%) had MSK manifestations and serologic findings related to RD after SARS-CoV-2 infection (Table 1). Considering these 36 patients (56% female), the median age at infection was 15 [12-16] years and the median time for serological or MSK manifestations after SARS-CoV-2 infection was 23 [15-40] days. All patients had asymptomatic (54%) or mild infection, and none required hospitalization.
Following infection, the main MSK manifestations were arthralgia (23/36, 64%), fatigue (16/36, 44%), myalgia (16/36, 44%) and acrocyanosis (8/36, 22%). The most frequent diagnosis identified were nonspecific musculoskeletal pain (NMKP: 11/36, 31%), perniosis (8/36, 22%), myositis (4/36, 11%) and connective tissue diseases (CTD: 3/36, 8%). Two patients (0.2%) had positive serologic findings (one with ANCA-PR3 and one with lupus anticoagulant), but no clinical manifestations related to these markers.
Five patients (14%) were hospitalized due to the severity of the developed RD: two with systemic lupus erythematosus (6%) and three with myositis (8%).
After a median follow-up of 15 [7.5-18] months, nearly all patients presented favorable outcomes. Regarding the patients who fulfilled diagnostic criteria of an inflammatory RD: four (11.1%) patients had complete remission, eight (22.2%) had remained with minimal disease activity and one (2.7%) with active disease. From the non-inflammatory patients, 11 (30.6%) had complete remission.
Conclusion: To the best of our knowledge, this is one of the few studies analyzing the MSK involvement induced by SARS-CoV-2 in a pediatric population. In this cohort, the MSK manifestations were uncommon (3.4%), and the most frequent were NMKP, perniosis, myositis and CTD. The manifestations had a wide spectrum of severity, from mild to potentially fatal, but were early identified, treated and 41.6% reached complete remission.
REFERENCES: [1] Gracia-Ramos AE. Cells. 2021.
[2] Cui,. Open Forum Infect Dis. 2022.
[3] Essien F. Ther Adv Chronic Dis. 2022.
[4] Luchetti Gentiloni MM. Front Immunol. 2022.
[5] Sacchi MC. Clin Transl Sci. 2021.
[6] Pascolini S. Clin Transl Sci. 2021.
Acknowledgements: NIL.
Disclosure of Interests: None declared.