

Background: Phase III clinical trials have shown that COVID-19 vaccines are safe and effective in healthy children. There are few studies in children with juvenile autoimmune disease (AID).
Objectives: The study aims to evaluate the immunogenicity and safety of the vaccine against COVID-19 in Juvenile JAID in the real life.
Methods: These data are from “Safety and efficacy on COVID-19 Vaccine in Rheumatic Disease” - SAFER study, a Brazilian multicentric prospective phase IV study to evaluate COVID-19 vaccine AID. Immunogenicity and adverse events (AE) were assessed, after 3 doses, in children compared to adults with AID. Inclusion criteria were fulfilling criteria according to international classification for AID. Exclusion criteria: pregnancy, previous severe AE to any vaccine and other immunosuppression causes. Stratification of post-vaccination AE was performed using a diary, filled out daily and returned at the end of 28 days of each dose. The IgG antibodies to SARS-CoV-2 spike receptor-binding domain by chemiluminescence were measured at baseline and after first and 2nd dose. The seropositivity was defined for titers ≥ 7 BAU/mL. The Kruskal-Wallis, post-hoc Dwass-Steel-Critchlow-Fligner pairwise comparisons tests, and multiple linear regression analysis were used. The p-value ≤0.05 was considered significant.
Results: A total of 123 volunteers with AID, 37 adults and 86 juvenile AID patients were included in the study.
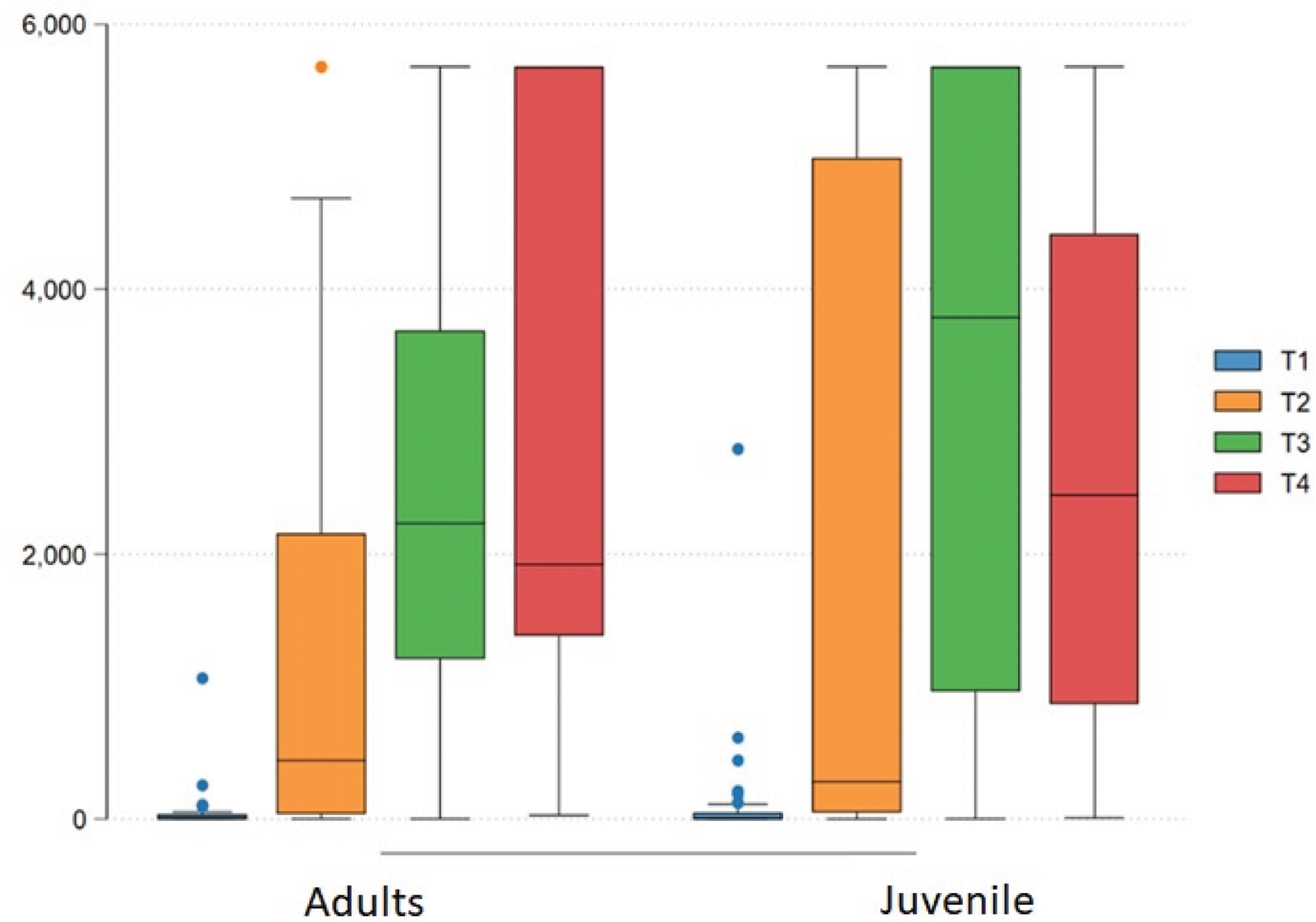
The juvenile group was aged < 18 years (adult 32.5 ±6 vs children group 14.5 ±1.7, p<0.01) and had fewer comorbidities (19 vs. 60%, p<0.01). The groups were homogeneous for sex (female 86% vs. 75%, p=0.23), ethnicity (Caucasians 47 vs. 43%, p=0.7) and degree of immunosuppression (high grade 62 vs. 60%, p=0.25). The frequency of diseases in adults was SLE= 56%, SpA= 5.41%, RA= 5.1%, others= 33.2%; and in children it was SLE= 38.4%, JIA= 41.8, others= 19.7%. The frequency of previous natural exposure to COVID-19 was higher in adults (30.6 vs. 8.1%, p<0.001). Both groups received BNT162b2 vaccine in the 1st. and 2nd. doses and in more than 90% in the 3rd. dose. The frequency of joint paint in post-1st dose (41 vs. 15%, p= 0.003) and in 2nd. dose (24 vs. 0%, p=0.003) was higher in adults. There was no difference in adverse events between the groups after the third dose. The groups were homogeneous for IgG-S titers at baseline. Patients with positive or negative IgG-S at baseline were analyzed separately. There was a significant increase (p<0.001) in IgG-S levels after the 1st, 2nd and 3th doses in the juvenile AID (0.71(0.28-35.07) vs. 443.04 (36.92-2155.77) vs. 2232.38 (1207.43-3688.45) vs. 1920.72 (1383.45-5680.00)), and in the adult group ((1.14(0.14-46.86) vs. 280.17 (49.70-4991.06) vs. 3787.14 (964.28-5680) vs. 2445.24 (868.12-4.418.19)). There was no difference between groups in the 4 times of comparison (p<0.05), regardless of the antibody level at baseline and the degree of immunosuppression. The seroconversion rate was 95% and 100% in both groups, after the 2nd and 3th dose.
Conclusion: BNT162b2 vaccine is effective, safe and induced high titles and seroconversion rate in juvenile AID, similar to adults, independent of immunosuppression, comorbidities or previous natural infection. Children showed more joint pain as AE than adults.
REFERENCES: NIL.
IgG-S measurement after vaccination against COVID-19 in baseline, 28 days after 1st dose, 2nd and 3th dose
| Total | Adults | Juvenile | P | |
|---|---|---|---|---|
| N=123 | N=37 | N=86 | ||
| IgG at inclusion, Median (IQR) | 0.85 (0.14-46.15) | 0.71 (0.28-35.07) | 1.14 (0.14-46.86) | 0.98 |
| IgG 28 days after 1st dose Median (IQR) | 326.96 (45.16-3887.32) | 443.04 (36.92-2155.77) | 280.17 (49.70-4991.06) | 0.33 |
| IgG 28 days after 2nd dose, Median (IQR) | 3189.32 (965.15-5245.62) | 2232.38 (1207.43-3688.45) | 3787.14 (964.28-5680.00) | 0.14 |
| IgG after 3th dose, Median (IQR) | 2287.78 (888.89-5680.00) | 1920.72 (1383.45-5680.00) | 2445.24 (868.12-4418.19) | 0.67 |
IgG-S measurement after vaccination against COVID-19 in T1(baseline, at inclusion), T2 (28 days after 1st dose), T3 (28 days after 2nd dose) and T3 (28 days after 3th dose).
Acknowledgements: We thank Safer Study Group, and the Brazilian Society of Rheumatology for supporting this study. This study was funded by Department of Science and Technology of Federal Government of Brazil.
Disclosure of Interests: None declared.