

Background: Juvenile idiopathic arthritis (JIA) may induce substantial morbidity and diminished quality of life, accompanied by a reduction in health-related quality of life and an increased susceptibility to enduring joint disability, persisting into adulthood. The contemporary proliferation of therapeutic agents, encompassing conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and biologic/targeted synthetic DMARDs (b/tsDMARDs), has facilitated efficacious disease management for numerous patients. However, this has concurrently engendered heightened intricacy in treatment decision-making.
Objectives: Systematic reviews (SRs) were conducted informing the clinical practical guidelines (CPG) for JIA with oligoarthritis or polyarthritis
Methods: The SR was implemented as a project of the Ministry of Health, Labor and Welfare’s research group in collaboration with the Japan College of Rheumatology, using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method. Seven key outcomes were selected: remission, achieving ACR ped 30 or a similar composite index, child-health assessment questionnaire disability index (C-HAQ DI), number of limited joints, serious adverse drug reactions/adverse events, serious infections, and treatment retention rate. The search period was from January 1990 until November 2022, and only articles written in English or Japanese were included. Patient, intervention, comparison, and outcome (PICO) from the CQs were abstracted, and PubMed, the Cochrane Library, and the Japan Centra Revuo Medicina databases were searched using search terms used in each of the CQs. Randomized controlled trials (RCTs) and RCT sub-analyses were adopted for efficacy. In the analysis of RCT, we examined the risk of bias using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). The certainty of evidence for each outcome was evaluated using the methods proposed by the GRADE working group. Recommendations were prepared based on the results of the SRs and structured abstracts of additional retrieved literature. Recommendations are agreed upon by a panel including patients, pediatric and non-pediatric rheumatologists, a nurse specialized in patient care with rheumatic diseases, guideline experts, and health economists using the Delphi method.
Results: Six CQs regarding efficacy and safety of medical treatment were evaluated, with CQ1 addressing methotrexate (MTX), CQ2 non-MTX csDMARDs, CQ3 glucocorticoids (GCs), CQ4 tumor necrosis factor (TNF) inhibitors, CQ5 interleukin (IL)-6 inhibitors, and CQ6 Janus kinase (JAK) inhibitors. The total number of candidate articles was initially 3,511 in PubMed, 1,698 in Cochrane, 395 in Japan Centra Revuo Medicina, and 16 others. From these sources, two RCTs for CQ1, three RCTs for CQ2, two post-hoc analyses of RCTs for CQ3, eight RCTs for CQ4, two RCTs for CQ5, and two RCTs for CQ6 were identified. The overall quality of evidence was moderate for CQ5, low for CQ6, and very low for CQ1, 2, 3, and 4. Afterward, six recommendations were developed (three strong ones and three conditional). The CPG has also been approved by the Pediatric Rheumatology Association of Japan.
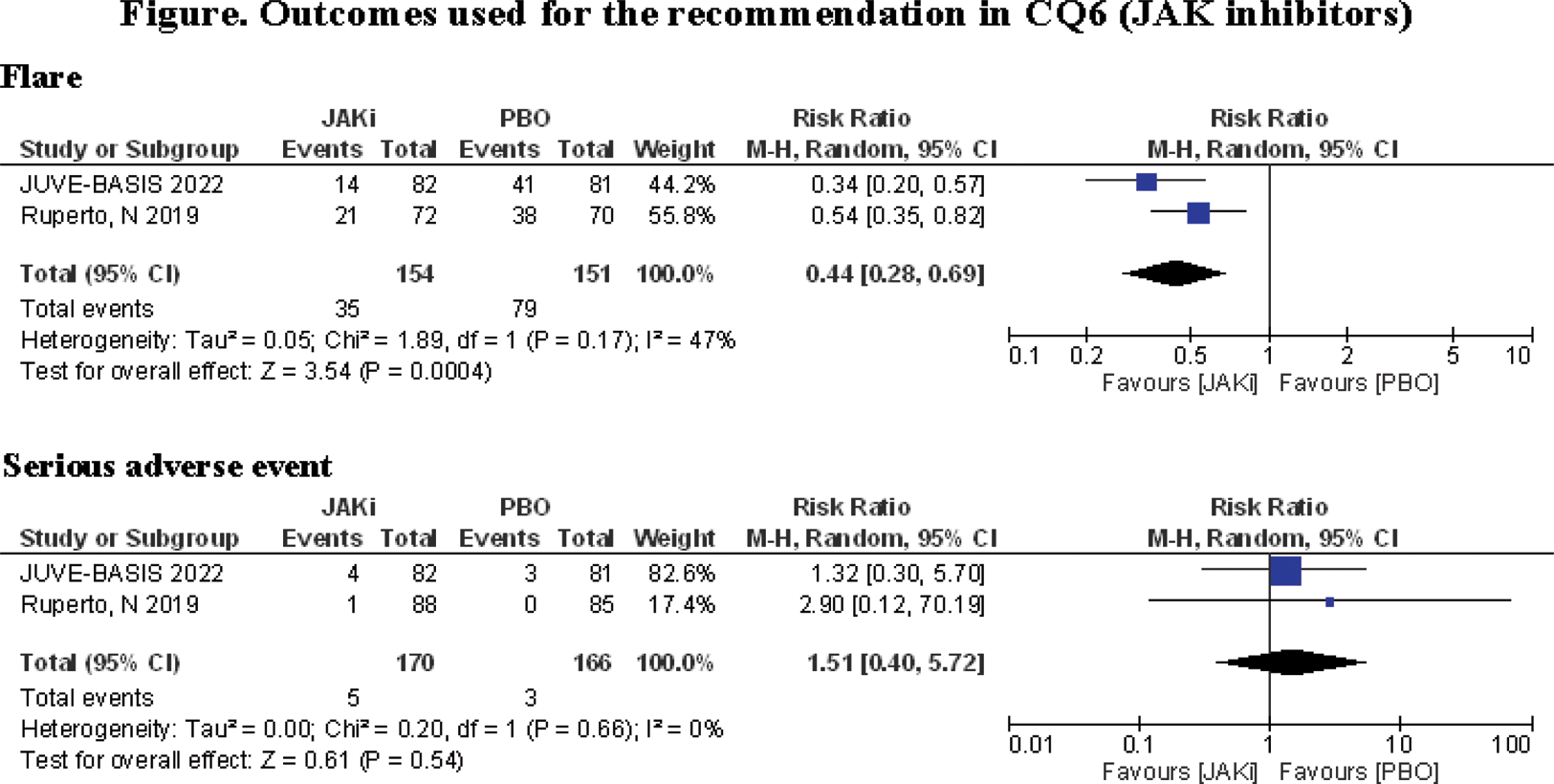
Conclusion: The SRs provided the necessary evidence therapeutic agents, including b/tsDMARDs, needed to develop a CPG for managing JIA with oligoarthritis or polyarthritis. We were the inaugural entity globally to undertake a comprehensive systematic review pertaining to JAK inhibitors. The CPGs are intended for healthcare professionals not limited to pediatrics, caregivers, and patients and their family members making treatment decisions. Including non-pediatric rheumatologists as panel members substantially contributed to establishing consensual care during the transition to adulthood.
REFERENCES: NIL.
Acknowledgements: We extend our deepest gratitude to the patient representatives, the Systematic Review (SR) team, the SR support committee members, and the members of Cochrane Japan for their cooperation in the development of this guideline.
Disclosure of Interests: Tomohiro Kawabe: None declared, Takako Miyamae Abbie Japan GK, Chugai Pharmaceutical Co., Pfizer Japan Inc., UCB Japan Co. Ltd., Nami Okamoto Abbie, Novartis Pharma, Eli Lilly, Asteras, Bristrol Myers Squib, Eli lilly as an advosory board menmber for international clinical study, Yuzaburo Inoue: None declared, Takasuke Ebato: None declared, Hitoshi Irabu: None declared, Hideto Kameda AbbVie, Asahi-Kasei, Bristol-Myers, Eisai, Eli Lilly, Janssen, Mitsubishi-Tanabe, Pfizer, Taisho and UCB, AbbVie, Amgen, Asahi-Kasei, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Taisho and UCB, Yuko Kaneko Pfizer, Gielad, Lilly, Eisai, Chugai, Tanabe-Mitsubishi, Asahi Kasei Pharma, Bristolo Myeres Squibb, Astellas, UCB, Abbvie, Taisho, Pfizer, Gielad, Lilly, Eisai, Chugai, Tanabe-Mitsubishi, Asahi Kasei Pharma, Hiroshi Kubo: None declared, Tomohiro Kubota: None declared, Kanako Mitsunaga: None declared, Ayako Nakajima: None declared, Kenichi Nishimura: None declared, Naoaki Ohkubo: None declared, Tomomi Sato: None declared, Yuko Sugita: None declared, Eiichi Tanaka ET has received lecture fees or consulting fees from AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., GlaxoSmithKline K.K., Kyowa Pharma Chemical CO., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical CO., Ltd., Nippon Kayaku Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd and Viatris Japan., ET has received research funding from Pfizer Inc. and UCB Japan Co. Ltd., Takayuki Tanaka: None declared, Nobuyuki Yajima: None declared, Masato Yashiro: None declared, Shingo Yamanishi: None declared, Ryo Yanai: None declared, Masaaki Mori AbbVie Japan, Asahikasei Pharmaceutical, Ayumi Pharmaceutical, Chugai Pharmaceutical, and UCB Japan, AbbVie Japan, Asahikasei Pharmaceutical, Ayumi Pharmaceutical, CSL Behring, Chugai Pharmaceutical, Japan Blood Products Organization, Nippon Kayaku, and UCB Japan, Yutaka Kawahito YK (Yutaka Kawahito) has received speaker’s fee from AbbVie GK, Asahi Kasei Pharma Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Corp., Bristol Myers Squibb Co., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., and Pfizer Japan Inc.., YK (Yutaka Kawahito) has received research grants from Asahi Kasei Pharma Corp., Ayumi Pharmaceutical Corp., AbbVie GK, ChugaiPharmaceutical Co. Ltd., Inc., Eisai Co., Ltd., Gilead Sciences, Inc., and Pfizer Japan Inc., Masayoshi Harigai Abbie Japan GK, Asahi Kasei Corp., Ayumi Pharmaceutical Co., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences Inc., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., and Teijin Pharma Ltd., AbbVie Japan GK, Asahi Kasei Corp., Ayumi Pharmaceutical Co., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Eisai Co., Ltd., Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., and Viatris Japan.