

Background: Tumor necrosis factor-alpha inhibitors (TNFi) are a standard in the treatment of juvenile idiopathic arthritis (JIA). There are currently three TNFi available for the treatment of polyarticular JIA (pJIA). Comparative data in children are scarce.
Objectives: To analyze baseline, effectiveness and safety parameters of tumor necrosis factor-alpha inhibitor first-line therapy in comparison
Methods: In this ongoing non-interventional study, baseline clinical characteristics, efficacy and safety parameters of TNFi first-line therapy with etanercept (ETA), adalimumab (ADA) or golimumab (GOL) were analyzed using the German Biologics in Pediatric Rheumatology Registry (BiKeR). Data from 2200 patients with pJIA/ extended oligo JIA (eoJIA) up to November 24 th 2023 were taken into account.
Results: ETA was the most frequently chosen primary TNFi with 1791 patients. ETA patients had a higher disease activity according to JADAS10 at baseline. Patients who primarily received ADA were more frequently diagnosed with eoJIA and less frequently with rheumatoid factor (RF)-positive pJIA. They were more frequently diagnosed with uveitis and positive for antinuclear antibodies (ANA). GOL initiating patients had less frequently eoJIA and were older at disease onset. They received concomitant methotrexate (MTX) more and steroids less frequently. No differences were observed in gender with a majority of female patients in all three groups (Table 1).
After 12 months, in all three cohorts, a significant reduction in the mean JADAS10 was achieved. With ETA/ADA/GOL, there was a decrease in mean JADAS10 from 15.4/12.5/14.6 to 3.8/3.1/2.7 and 3.4/2.8/2.3 after 24 months respectively. After 12 months of treatment, 61.7/70.2/68.6% achieved JADAS 10 remission and 78.4/84.4/85.7% attained minimal disease activity. PedACR90 scores were reached in 45.6/40.9/52.8% in the three cohorts. After 24 months of treatment, efficacy data showed consistent or improved results with 63.1/68.8/55.6% achieved JADAS remission and 80.4/85.2/83.3% attained minimal disease activity and PedACR scores in 51.0/41.3/44.4%.
Reports of SAEs, serious infections (SI) and treatment discontinuations are shown in Table 1, along with efficacy data. SAEs and serious infections were rare overall and occurred at the expected frequency. Of note, the highest rate of uveitis recurrence was in the ADA cohort, which also had the highest rate of prior uveitis. Treatment discontinuations were comparable in all three cohorts at 61.8/59.2% and 59.2%, respectively. Lack of efficacy was the most common reason for discontinuation at 21.3/20.6/20.4%.
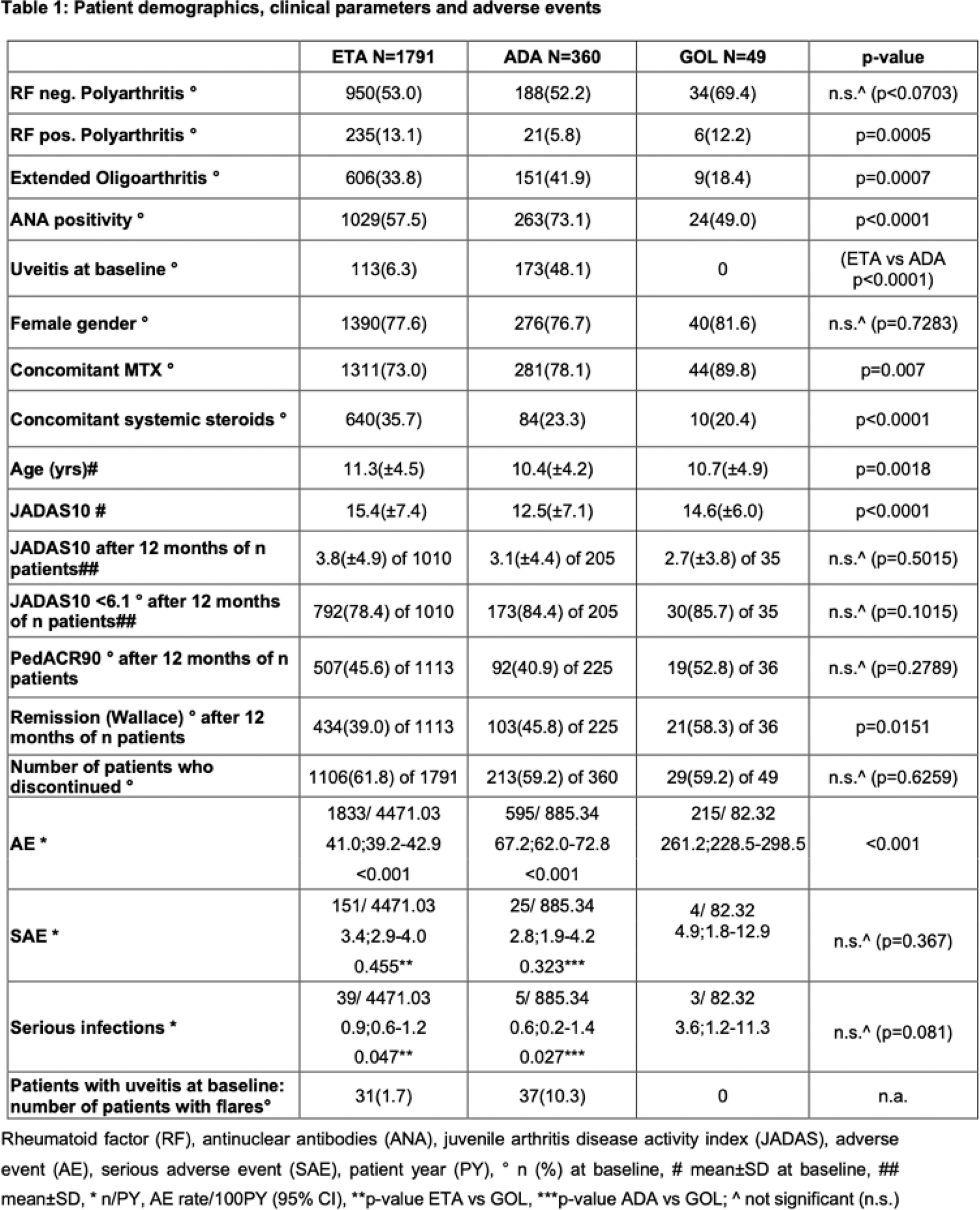
Conclusion: Numerous patient characteristics influence the choice of TNFi for the treatment of polyarticular JIA. While ETA remains the most frequently chosen first-line biologic, ADA is preferred in the presence of uveitis. In accordance with German regulations, GOL is concomitantly prescribed with methotrexate therapy. Efficacy, tolerability and safety appear to be comparable.
REFERENCES: NIL.
Acknowledgements: The authors thank Rainer Berendes, Michael Borte, Maria Fasshauer, Ivan Földvari, Tilman Geikowski, Hermann Girschick, Jürgen Grulich-Henn, Johannes-Peter Haas, Maria Haller, Boris Hügle, Bernd-Ulrich Keck, Hans Kössel, Jasmin Kümmerle-Deschner, Rolf-Michael Küster, Kirsten Minden, Nils Onken, Prasad Oommen, Jürgen Quietzsch, Bettina Rogalski, Michael Rühlmann, Angelika Thon, Ralf Trauzeddel, Andreas Urban, Frank Weller-Heinemann for contributing to the BIKER-Registry and all patients and their families for participating in this observation.
Disclosure of Interests: Angela Zimmer: None declared, Ariane Klein: None declared, Frank Dressler Abbvie, Novartis, Pfizer, Novartis, Mylan, Daniel Windschall Novartis, Pfizer, Abbvie, MEDAC, Roche, Sobi, Novartis, Roche, Pfizer, Abbvie, Normi Brueck: None declared, Sonja Mrusek: None declared, Toni Hospach Novartis, SOBI, Novartis, SOBI, Markus Hufnagel Novartis, Kirsten Minden Pfizer, Novartis, medac, Gerd Horneff Pfizer, Roche, MSD, Sobi, GSK, Sanofi, AbbVie, Chugai, Bayer, Novartis, MSD, Lilly, Pfizer, Roche, MSD, AbbVie, Chugai, Novartis.