

Background: Golimumab (GOL) is approved for treatment of polyarticular juvenile idiopathic arthritis (pJIA) in patients two years and older. Data on long-term safety of GOL in this indication are limited.
Objectives: To assess long-term safety and effectiveness of GOL in patients with pJIA.
Methods: In this ongoing non-interventional observational study, clinical characteristics, disease activity and safety parameters were analysed using data from the German Biologics in Paediatric Rheumatology (BiKeR) registry. Patients treated with GOL were frequency matched by body weight to patients treated with alternative tumor necrosis factor inhibitors (aTNFi) or methotrexate (MTX, biologic–naïve).
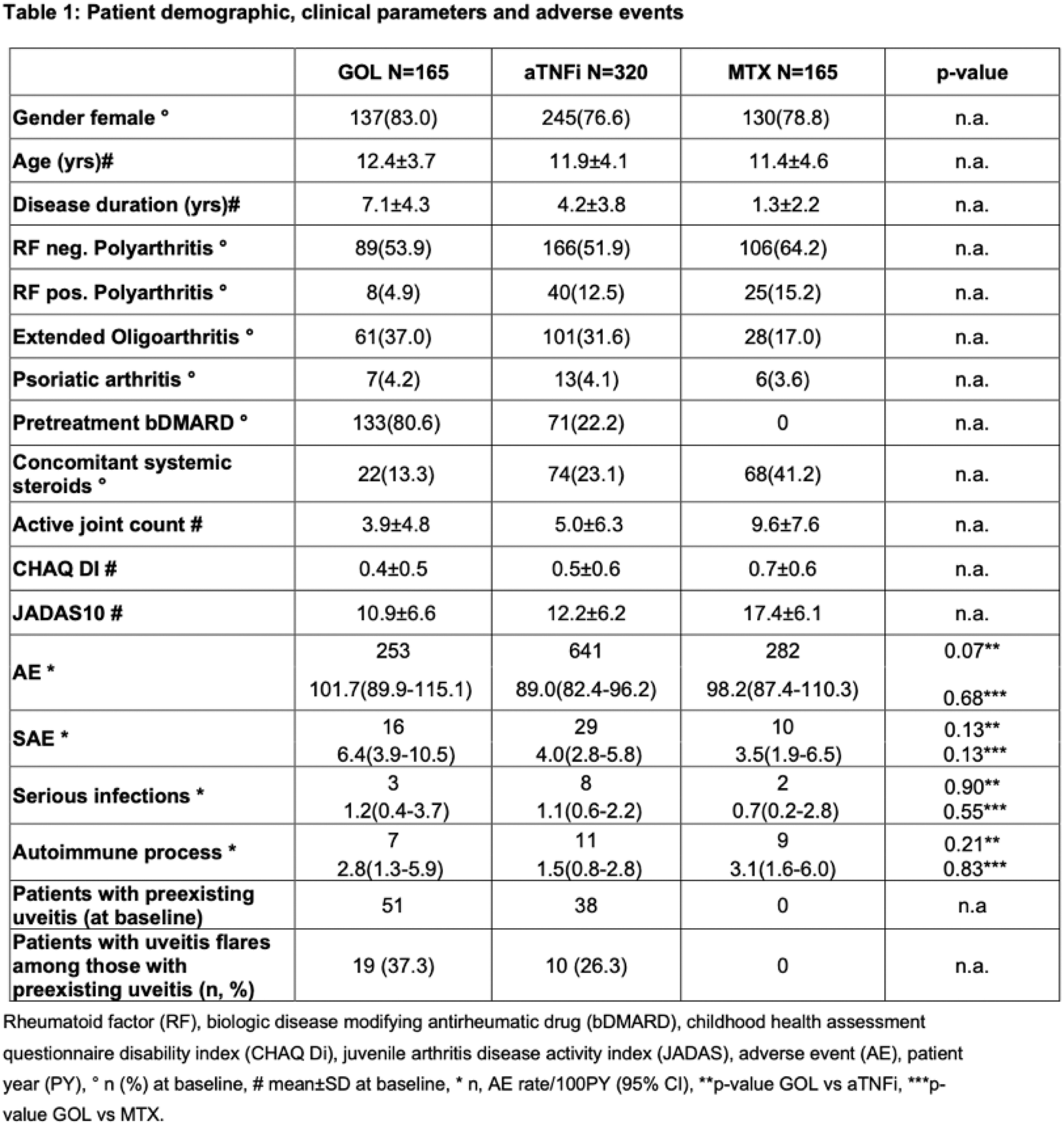
Results: A total of 650 patients with pJIA were evaluated: 165 treated with GOL, 320 treated with aTNFi and 165 treated with MTX. Rheumatic factor (RF)- negative polyarticular JIA was the most common JIA category (361; 56%) followed by extended oligoarthritis (190; 29%), RF-positive polyarticular JIA (73; 11%) and psoriatic arthritis (26; 4%). GOL-cohort patients had lower concomitant systemic steroid use, a longer disease duration and were previously more exposed to other biologic agents than the aTNFi cohort (Table 1). Baseline disease activity (measured by number of active joints and JADAS10) of the GOL cohort was similar compared to the aTNFi cohort, and lower compared to the MTX cohort (Table 1).
The clinical effectiveness of GOL in pJIA was observed in 3 to 6 months post-baseline and was generally sustained through observation with a decrease in the mean JADAS-10 from 10.9 (±6.6) (baseline) to 3.5 (±4.3) at 24 months (38/165 GOL patients accrued 24 months follow-up). By 24 months, a JADAS10 minimal disease activity (JADAS10 ≤6) was reached by 81.6% of patients and 55.3 % of patients showed inactive disease (JADAS10 ≤2.7). The PedACR 30/50/70/90 response rates at 24 months were 73.7/65.8/63.2/50.0% respectively.
In the GOL cohort, 16 SAEs were reported, compared to 29 in the aTNFi and 10 in the MTX cohort (Table 1). There were three serious infections in the GOL cohort, eight in the aTNFi and two in the MTX cohort. A few incident autoimmune events were reported: 7 in the GOL cohort (2 uveitis, 3 psoriasis, 2 inflammatory bowel disease (IBD)), 11 in the aTNFi cohort (6 uveitis, 3 psoriasis, 2 IBD) and 9 in the MTX cohort (8 uveitis, 1 celiac disease) (Table 1). Among the patients with preexisting uveitis at baseline, 19/51 in the GOL cohort and 10/38 in the aTNFi cohort had uveitis flares. There were no cases of preexisting uveitis in the MTX-cohort (Table 1). No malignancies were reported in any cohort.
Conclusion: Interim results from the BiKeR registry in pJIA show an acceptable safety profile of GOL therapy, comparable to that of aTNFi or MTX. AE and serious infection rates between the three cohorts were comparable. No new safety signals were identified. Data indicate long term effectiveness of GOL in the treatment of pJIA.
REFERENCES: NIL.
Acknowledgements: The authors thank Zhiping Huang, Deanna Hill, Rainer Berendes, Michael Borte, Maria Fasshauer, Ivan Földvari, Tilman Geikowski, Hermann Girschick, Jürgen Grulich-Henn, Johannes-Peter Haas, Maria Haller, Boris Hügle, Bernd-Ulrich Keck, Hans Kössel, Jasmin Kümmerle-Deschner, Rolf-Michael Küster, Kirsten Minden, Nils Onken, Prasad Oommen, Jürgen Quietzsch, Bettina Rogalski, Michael Rühlmann, Angelika Thon, Ralf Trauzeddel, Andreas Urban, Frank Weller-Heinemann for contributing to the BIKER-Registry and all patients and their families for participating in this observation.
Disclosure of Interests: Angela Zimmer: None declared, Ariane Klein: None declared, Frank Dressler Abbvie, Novartis, Pfizer, Novartis, Mylan, Daniel Windschall Novartis, Pfizer, Abbvie, MEDAC, Roche, Sobi, Novartis, Roche, Pfizer, Abbvie, Normi Brueck: None declared, Sonja Mrusek: None declared, Toni Hospach Novartis, SOBI, Novartis, SOBI, Markus Hufnagel Novartis, Kirsten Minden Pfizer, Novartis, Medac, Gerd Horneff Pfizer, Roche, MSD, Sobi, GSK, Sanofi, AbbVie, Chugai, Bayer, Novartis, MSD, Lilly, Pfizer, Roche, MSD, AbbVie, Chugai, Novartis.