

Background: Five hundred million people worldwide are affected by osteoarthritis (OA), yet many patients still have unmanaged pain due to our incomplete understanding of the pathways driving OA pain. Clinically, aspects of inflammation such as synovitis and effusion have been associated with pain sensitization in OA [1], and current evidence suggests that innate immune pathways play an important role in OA inflammation and pain [2]. Mice in which the TLR co-receptor CD14 was knocked-out were shown to have late stage protection against cartilage damage and preserved locomotor activity in the DMM mouse model [3]. In OA, a correlation between synovial fluid levels of soluble CD14 and pain severity has been observed.
Objectives: The goals of this study were to investigate whether CD14 contributes to pain-related behaviors in the partial medial meniscectomy (PMX) model of OA as well as whether CD14 contributes to knee hyperalgesia in response to intra-articular injection of TLR ligands.
Methods: All animal procedures were approved by an IACUC committee. Experiment one : At 12 weeks of age, Cd14 -/ - and C57BL/6 WT male mice underwent sham or PMX surgery of the right knee. A blinded observer assessed knee hyperalgesia and hind paw mechanical allodynia every four weeks; weight bearing was assessed at baseline, 6 and 10 weeks post PMX . Experiment 2: Naïve male Cd14 -/ - mice and WT mice were used to determine whether CD14 is protective against knee hyperalgesia induced by intraarticular injection (i.a.) of TLR agonists. Mice were injected i.a. with either saline (3 μL), LPS (3 μg in 3 μL), or Pam3CSK4 (3 μg in 3 μL). Knee hyperalgesia was assessed prior to injection, two hours and four hours post injection. At 6 h post injection mice were taken down and ipsilateral L3-L5 dorsal root ganglia (DRG) were removed, placed in Trizol, and RNA was extracted for qPCR using primers for Gadph and Ccl2 . Experiment 3 : In vitro DRG cultures were performed using L3-L5 DRGs collected from either 2 Cd14 -/ - or from 2 WT mice. On day three of the culture, cells were stimulated for 24 h with either vehicle, LPS, or Pam3CSK4 and supernatants were used for a CCL2 ELISA.
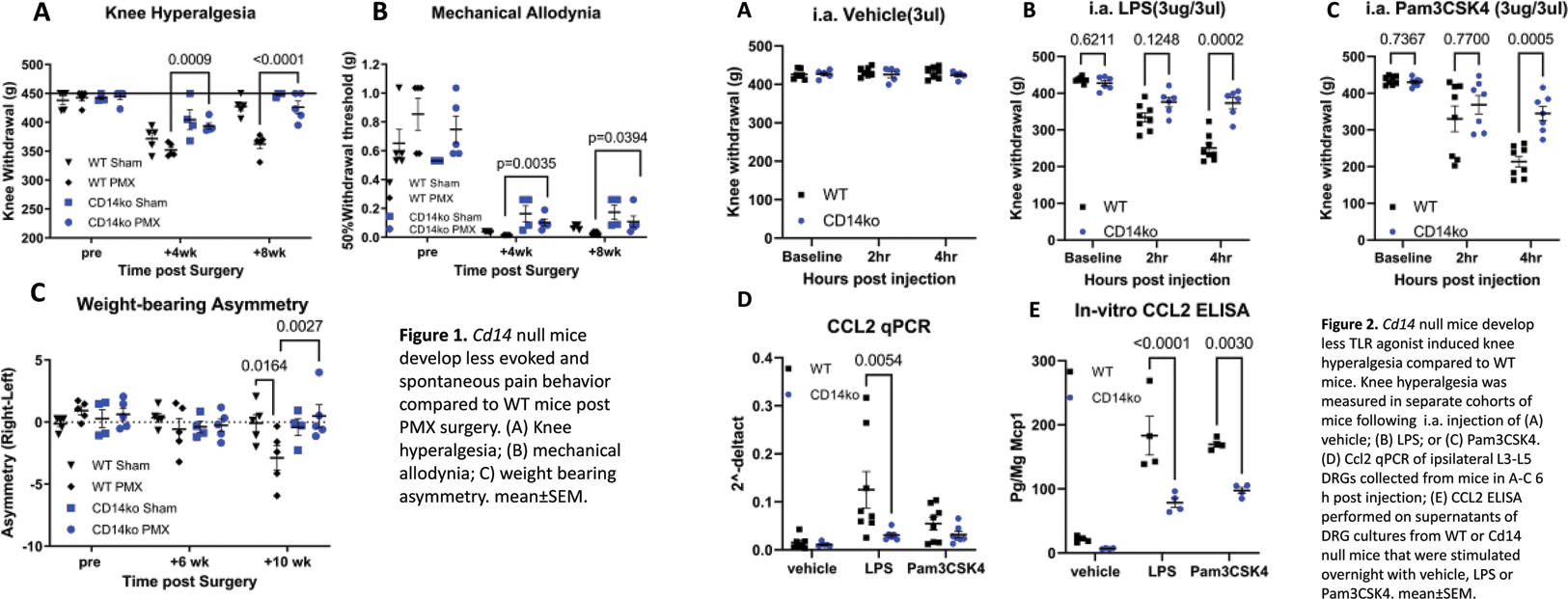
Results: Experiment one : Mice receiving PMX surgery (n=5 Cd14 -/ - , n=5 WT) and sham (n=4 Cd14 -/ - , n=5 WT) were assessed for knee hyperalgesia and hind paw mechanical allodynia until eight weeks post-surgery. Cd14 -/ - mice had significantly less knee hyperalgesia in comparison to WT mice 4 and 8 weeks post PMX (p=0.0009, p<0.0001, 2-way ANOVA, Figure 1A). A similar trend was found when assessing mechanical allodynia (Figure 1B). Finally, WT PMX mice developed weight-bearing deficits in the operated limb by 10 weeks post-surgery compared to WT sham mice (p=0.0164), while Cd14 -/ - mice did not (p=0.9194) (Figure 1C). Experiment two : Naïve mice were intra-articularly injected with either saline, LPS, or Pam3CSK4. Intra-articular injection of saline did not induce knee hyperalgesia (Figure 2A). In contrast, LPS-induced knee hyperalgesia developed over the course of 4 hours in WT mice (n=8), but to a lesser extent in Cd14-/ - mice (n=6) (p=0.0002, 2-way ANOVA) (Figure 2B). A similar pattern was observed upon injection of the TLR2 ligand, Pam3CSK4 (Figure 2C). By 6 hours post injection of LPS, WT DRGs had higher levels of Ccl2 transcript levels compared to Cd14 -/- mice (p=0.0054, 2-way ANOVA) (Figure 2D). To confirm qPCR results, a separate experiment was performed by culturing L3-L5 DRG cells obtained from WT or Cd14 -/ - mice. In this case, stimulation with both LPS and Pam3CSK4 induced increased protein production of CCL2 in WT mice compared to vehicle (p<0.0001, p<0.0001), while Cd14 -/ - cells produced lower amounts of CCL2 compared to WT in response to both LPS (p=0.0003) and to Pam3CSK4 (p=0.01) (Figure 2E).
Conclusion: Cd14 -/ - mice develop less mechanical sensitization in an additional model of OA and in response to direct injection of TLR ligands into the knee joint. This protection might be partially mediated by reducing the amount of CCL2 (a pro-algesic chemokine) produced.
REFERENCES: [1] Neogi et al, Arthritis Rheumatol. 2016;68(3):654-61; 2. Miller RJ et al, Osteoarthritis Cartilage. 2020;28(5):562-571; 3. Sambamurthy et al, PLoS One. 2018;13(11):e0206217.
Acknowledgements: R01AR077019 (REM), R01AR064251, R01AR060364, P30AR079206 (AM), F31AR083277 (NA), R01AR075737, VA I01BX004912 (CRS)
Disclosure of Interests: None declared.