

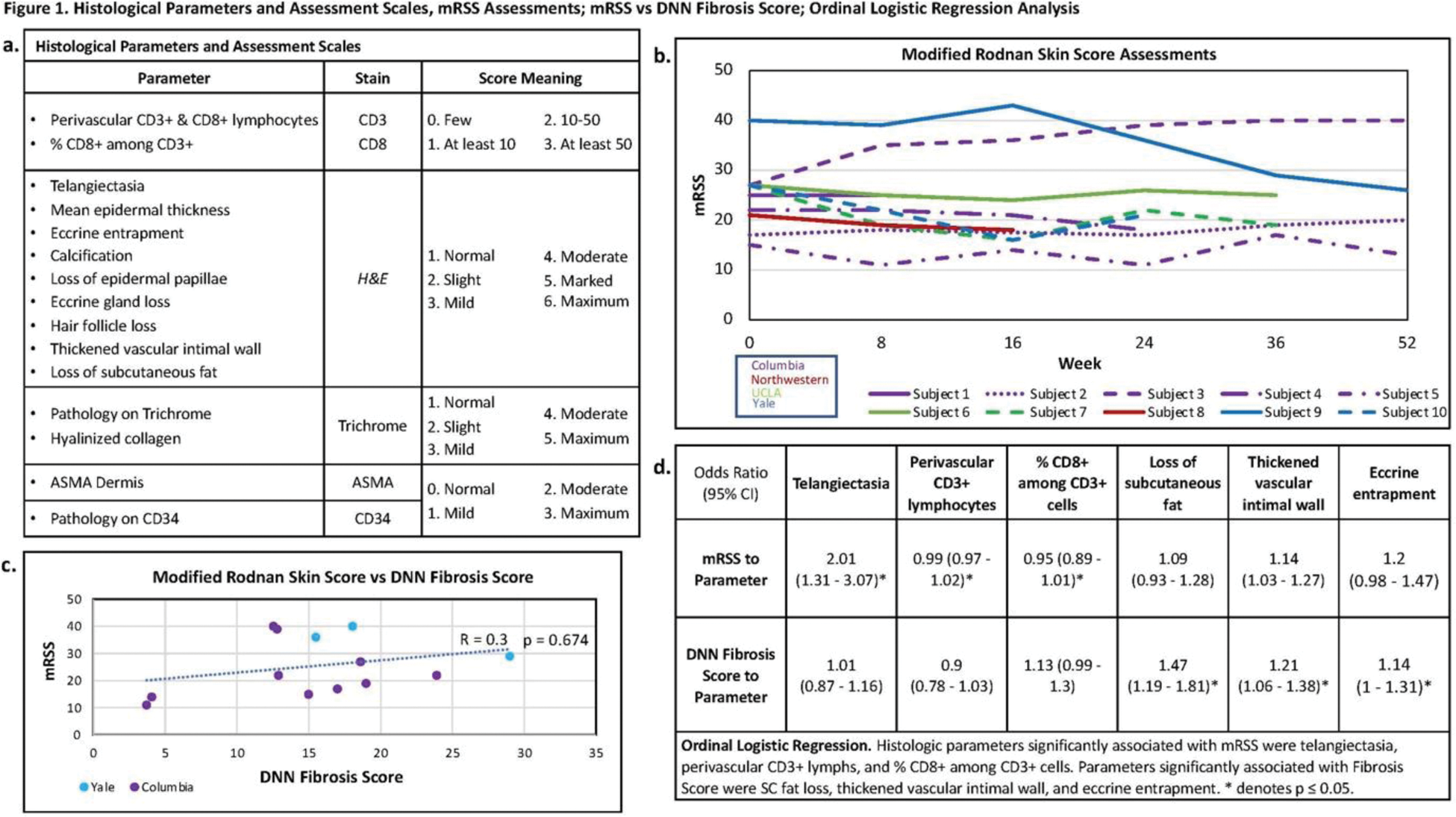
Background: We published proof-of-principle study results demonstrating the potential utility of computer vision (Deep Neural Network/DNN) methods applied to trichrome-stained skin biopsy sections from patients with systemic sclerosis (SSc) as a novel quantitative skin outcome. Briefly, an DNN algorithm (AlexNet) was applied to each of one hundred randomly selected image patches (0.16mm 2 ) to generate 4,096 quantitative image features (QIFs)/ image patch (409,600 QIFs/ section). A linear regression model comprised of QIFs (predictor variables) was generated that predicted the modified Rodnan Skin Score (mRSS) termed ‘DNN Fibrosis Score’. Others published 16 SSc-associated histological variables (Figure 1a) (Van Praet 2011).
Objectives: The present study compared real world ‘DNN Fibrosis Score’ changes with histopathologic and modified Rodnan Skin Score (mRSS) changes for clinical trial participants.
Methods: Ten adults with early (≤ 6 y) diffuse cutaneous SSc (15 ≤ mRSS ≤ 35) in a belumosudil (ROCK2 inhibitor, 200 mg PO BID) open-label trial had mRSS and 4mm, dorsal arm skin biopsies performed at weeks (W) 0, 24, and 52. Biopsies were stained with CD34, CD3, CD8, alpha smooth muscle actin (ASMA), H&E , and trichrome. Two blinded dermatopathologists assessed biopsies for 16 histopathological parameters. CD3+, CD8+ cells were counted, and ordinal scales were used to score CD34, ASMA, and relative SSc severity on H&E and trichrome-stained slides (Figure 1a). As above, AlexNet was applied to image patches to generate QIFs, and a linear regression model to predict mRSS was generated (‘DNN Fibrosis Score’). ‘DNN Fibrosis Scores’ were compared to mRSS (ρcorrelation). Histopathologic parameters were compared to mRSS or DNN Fibrosis Scores using logistic regression models where odds ratios and p-values were used to show the relationship between DNN Fibrosis Scores or mRSS changes and histological parameter changes (Figure 1d). A p≤0.05 was considered significant.
Results: Ten participants were enrolled, and the median (interquartile range/IQR) mRSS change between 0 - 52W was -2.5 (-11 to 7.5) (Figure 1b). Due to fixation issues, only five patients had paired biopsies available (Figure 2). The median/IQR DNN Fibrosis Score change (W0 - last follow-up) was -6 (-10.5 to 6.5) (Figure 1b). Of the histopathological scores, subcutaneous (SC) fat loss (p-value = 0.012), eccrine entrapment (p-value = 0.008), % CD8+ among CD3+ cells (p-value = 0.006) changed most during treatment. The correlation between mRSS and DNN Fibrosis Score for the five paired biopsies was 0.18, p-value =0.674 (weaker correlation at higher mRSS] (Figure 1c). Per 1-unit mRSS change, the histopathological parameter odds ratio (OR); p-values were: telangiectasia =2.01; 0.001, perivascular CD3+ =1.03; 0.015, and % of CD8+ among CD3+ =1.08; 0.031 (Figure 1d). Likewise, per 1-unit DNN Fibrosis Score change, OR; p-values for histopathological parameters were: hyalinized collagen =1.1; 0.00033, SC fat loss =1.47; 0.00033, thickened vascular intimal wall =1.21; 0.005, and eccrine entrapment =1.14; 0.046 (Figure 1d).
Conclusion: In this novel analysis to explore the potential utility of artificial intelligence applied to stained skin biopsies from patients with SSc as a quantity, the DNN Fibrosis Score mirrored changes in the mRSS and was sensitive to histopathologic changes. Belumosudil treatment was associated with non-clinically important mRSS improvement that mirrored decreases in telangiectasia, perivascular CD3+, and % CD8+ among CD3+ cells. The weak correlation between mRSS and DNN-Fibrosis Score contrasted with our previous findings that we attribute to batch effects from staining protocols between our published and our current analysis which may be overcome with analyses of larger cohorts.
Histopathology assessment and Belumosudil open-label trial results. a. Ordinal scales for histopathology. b. Subject’s mRSS W0 to last follow-up. c. Scatter plot of DNN Fibrosis Score and mRSS. d. Logistic regression comparing DNN Fibrosis Score to histologic parameters.
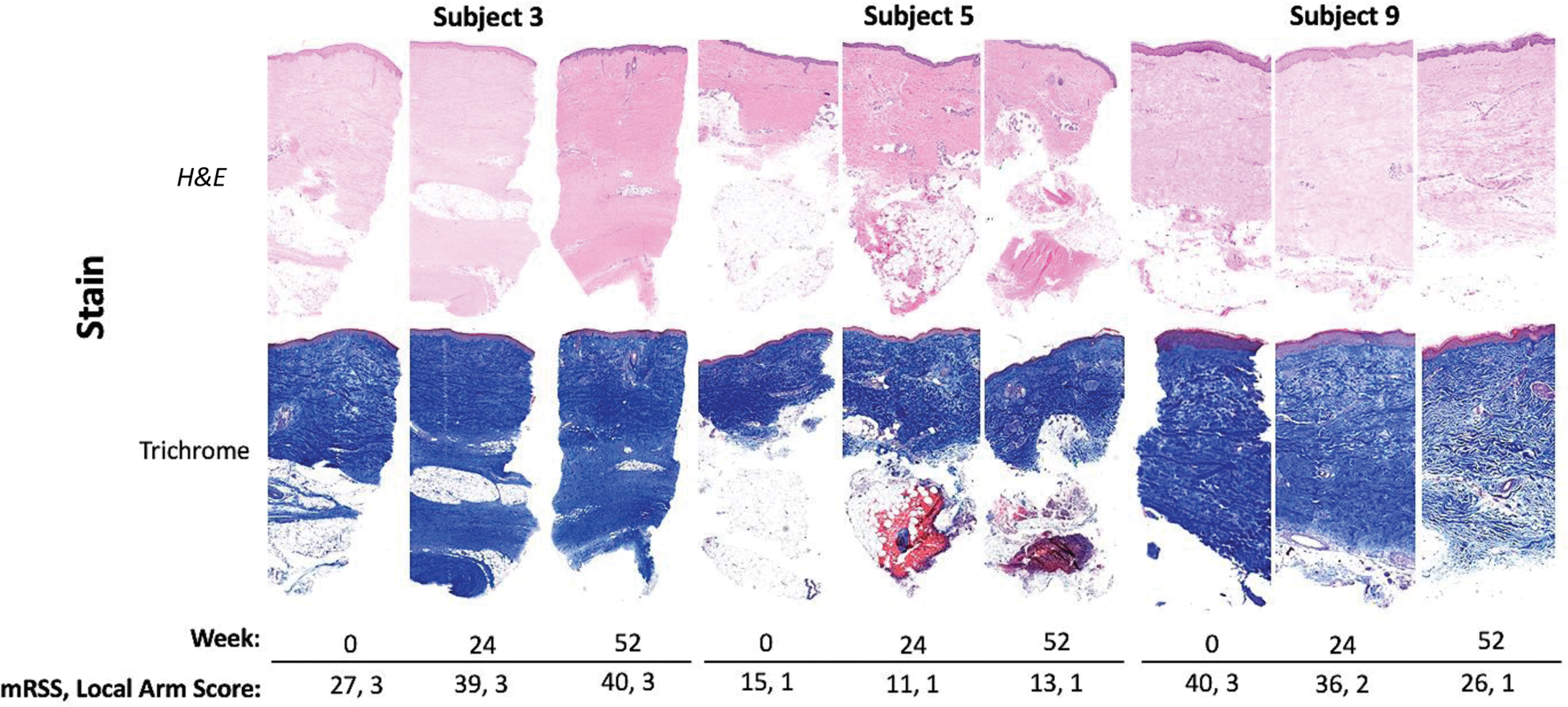
Stained skin biopsy slide images with corresponding mRSS. Stained sections from participants with the largest mRSS changes (40x).
REFERENCES NIL. Acknowledgements: Kadmon Corporation
Disclosure of Interests: Baran Gunes: None declared, Lucy Duran Camacho: None declared, Shawn Cowper: None declared, Gauri Panse: None declared, Nicolas Page: None declared, Elizabeth Bundschuh: None declared, Alyssa Williams: None declared, Mary Carns: None declared, Kathleen Aren: None declared, Niki Pradhan: None declared, Elana Bernstein Boehringer Ingelheim, Boehringer Ingelheim, Pfizer, Sarah Fantus: None declared, Elizabeth Volkmann Boehringer Ingelheim, Boehringer Ingelheim, GSK, CSL Behring, Boehringer Ingelheim, Kadmon, Horizon, Prometheus, Heather Bukiri: None declared, Chase Correia: None declared, Francis Wilson AstraZeneca, Amgen, and Vifor Pharma., Seamus Mawe: None declared, J. Matthew Mahoney: None declared, Rui Wang Sanofi, Monqiue Hinchcliff Kadmon Corporation and Boehringer Ingelheim.