

Background: Nailfold capillaroscopy plays a key role in the assessment of patients with Raynaud’s phenomenon (RP). Normal capillaries, as found in primary RP, are reassuring. Conversely abnormal capillaries (widened capillary loops, low capillary density) are predictive of a systemic sclerosis (SSc)-spectrum disorder. A recent UK survey [1] suggested that major contributors to many rheumatologists not performing capillaroscopy themselves were lack of equipment and lack of expertise in image interpretation.
Objectives: To investigate the hypothesis that accurate, fully automated analysis of nailfold capillaries can be obtained not only with specialist videocapillaroscopy systems but also with hand-held, low cost (USB) microscopes.
Methods: Four nailfolds (middle and ring fingers of each hand) were examined in patients with SSc, patients with primary RP, and healthy control subjects using two different capillaroscopy systems: a purpose-built nailfold videocapillaroscopy (NVC) system [2] (cost of parts ~8750 euros) and a commercially available handheld USB microscope (cost ~600 euros). For each nailfold, in-house acquisition software was used to record video sequences using the two systems. For the NVC system the software auto-generated a nailfold mosaic from the video [2], while for the USB, the best 5 frames were selected using a fully automated frame quality assessment model (no human input required). From the mosaics/frames obtained, a deep learning system [3] computed the following metrics for each nailfold: mean and maximum capillary widths (in um), mean shape score, and vessel density (per mm). These were averaged across all fingers to compute metrics per participant, and using a logistic regression model a participant ‘SSc-score’ was computed. Receiver operator characteristic (ROC) curves were derived to determine discrimination accuracy (positive SSc vs negative ‘non-SSc’) for each capillaroscopy system. For each ROC two summary performance measures were computed: the area under the curve (AUC: 50% = random classification, 100% = perfect classification), and the sensitivity and specificity at the point of equal error rate (EER).
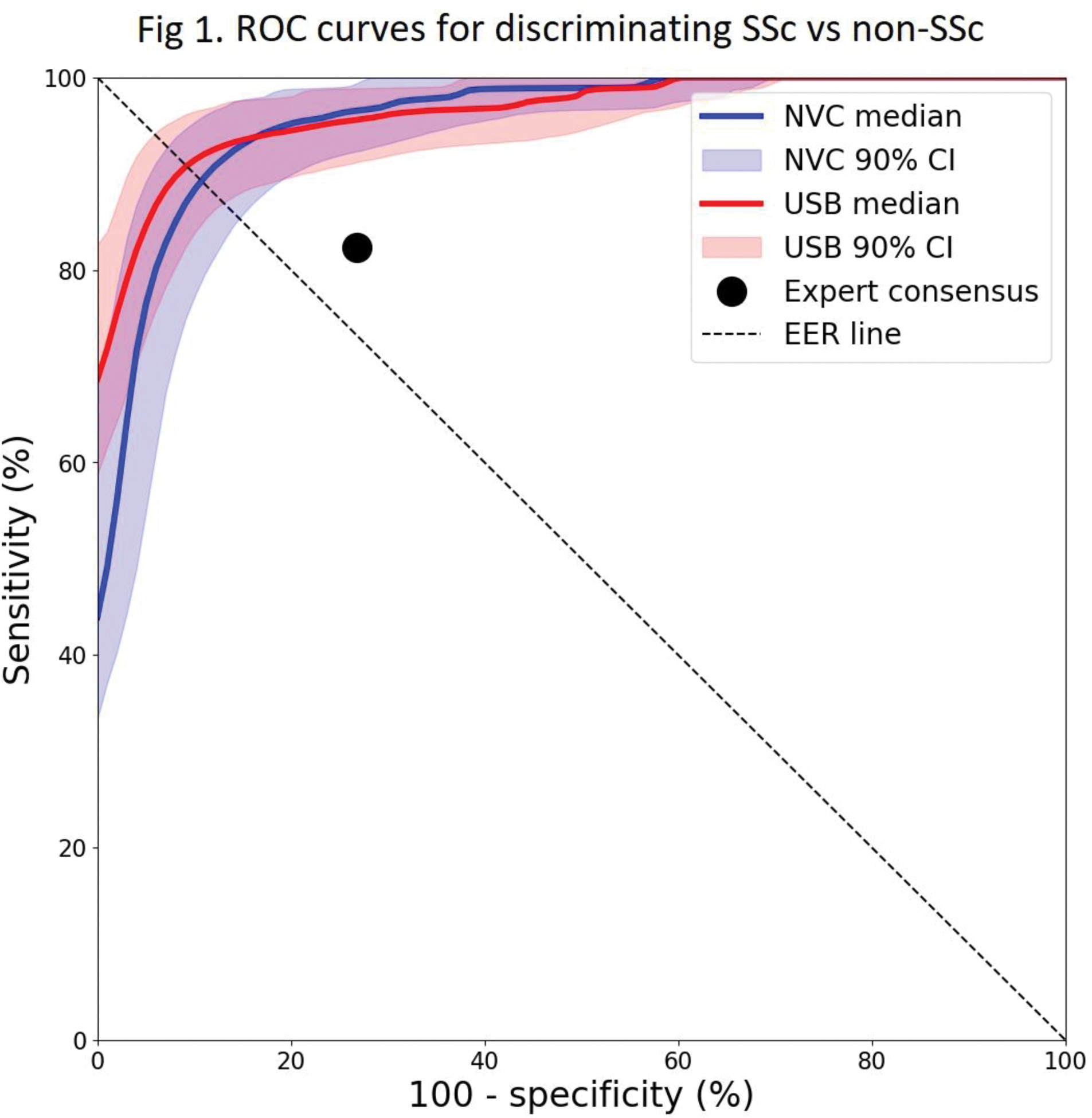
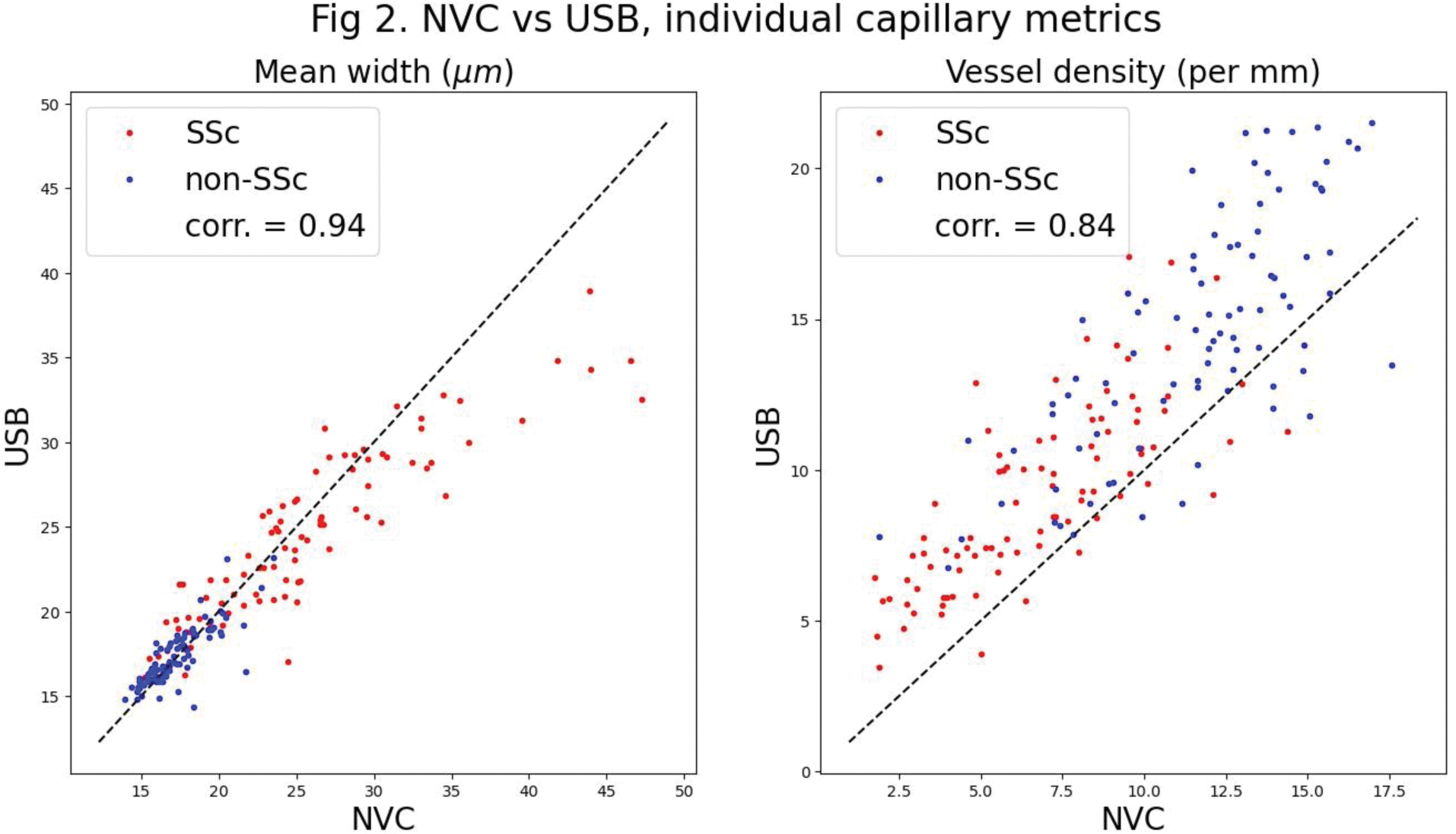
Results: 92 patients with SSc and 90 ‘non-SSc’ participants (with either primary RP [n= 11] or healthy control subjects [n= 79]) were recruited, most of whom (88 SSc and 87 non-SSc) were examined using both the capillaroscopy systems. Results for AUCs (median, 5 th -95 th centile) were: NVC 96% (93-98%), USB 96 % (94-98%), and for EER sensitivities/specificities: NVC 89% (85-93%), USB 91% (87-94%). Comparison to expert consensus opinion (sensitivity 82%, specificity 73%) [3] is shown in Figure 1. Participant-level metrics for the NVC vs USB systems respectively were as follows: mean width (um) SSc 25.76 vs 24.59, non-SSc 17.31 vs 17.40; maximum width (um) SSc 45.44 vs 43.24, non-SSc 26.57 vs 26.64; mean shape score SSc 0.30 vs 0.39, non-SSc 0.53 vs 0.51; capillary density (per mm) SSc 4.28 vs 6.52, non-SSc 6.72 vs 7.88. Figure 2 shows scatter-plots for mean width and density.
Conclusion: Fully automated analysis using a low-cost hand-held device achieved similar results to a high-specification device with automated mosaicking, and these results were at least as good as expert opinion. These results confirm, in a large dataset, our previous finding that automated analysis can be applied in low-cost microscopes. An important new finding is that high diagnostic performance is achievable without any human input to frame/image selection, removing subjectivity. Low-cost systems and automated analysis remove two of the barriers to rheumatologists using nailfold capillaroscopy in the out-patient clinic, and should facilitate early diagnosis of SSc.
REFERENCES: [1] Eden M, et al. Rheumatology 2023;
[2] Berks M, et al. MICCAI 2016;
[3] Gurunath Bharathi P, et al. Rheumatology 2023;
Acknowledgements: This work was funded by the National Institute for Health and Care Research (NIHR)
Disclosure of Interests: Michael Berks: None declared, Praveen Gurunath Bharathi: None declared, Andrea Murray Arena, Gesynta Pharma, Graham Dinsdale: None declared, Joanne Manning: None declared, Ariane L. Herrick Janssen, Arena, Boehringer Ingelheim, Camurus, CSL Behring, Galderma, Gesynta Pharma, Chris Taylor: None declared.