

Background: Interstitial lung disease (ILD) is the foremost contributor to mortality in systemic sclerosis (SSc). High-resolution computed tomography (HRCT) remains the gold standard for ILD diagnosis. While previously recommended for all patients at the time of SSc diagnosis, HRCT screening is now conditionally advised by the 2023 ACR guideline for managing ILD across rheumatic diseases, explicitly targeting patients at increased risk of developing ILD. The impact of not screening all SSc patients for ILD has so far not been assessed in detail.
Objectives: To explore the impact of HRCT screening on ILD identification, ILD progression, and survival across two countries with differing screening approaches.
Methods: This multicenter study involved 314 Romanian SSc (Ro-SSc) patients from “Sfanta Maria” Clinical Hospital in Bucharest (with non-universal HRCT-ILD screening) and 905 Norwegian SSc (Nor-SSc) patients from Oslo University Hospital (with routine HRCT screening since 2000). The study aimed to assess the prevalence of ILD and factors associated with HRCT conduction over three periods: 2000-2010, 2010-2020, and 2020-2023. Baseline and last available follow-up pulmonary function tests (PFTs) were retrieved. ILD progression was categorized based on forced vital capacity (FVC) decline as severe (>10% FVC decline) and moderate (5-10% FVC decline). We defined the presence of significant progression in the absence of HRCT as likely ILD. Logistic and Cox regression were conducted.
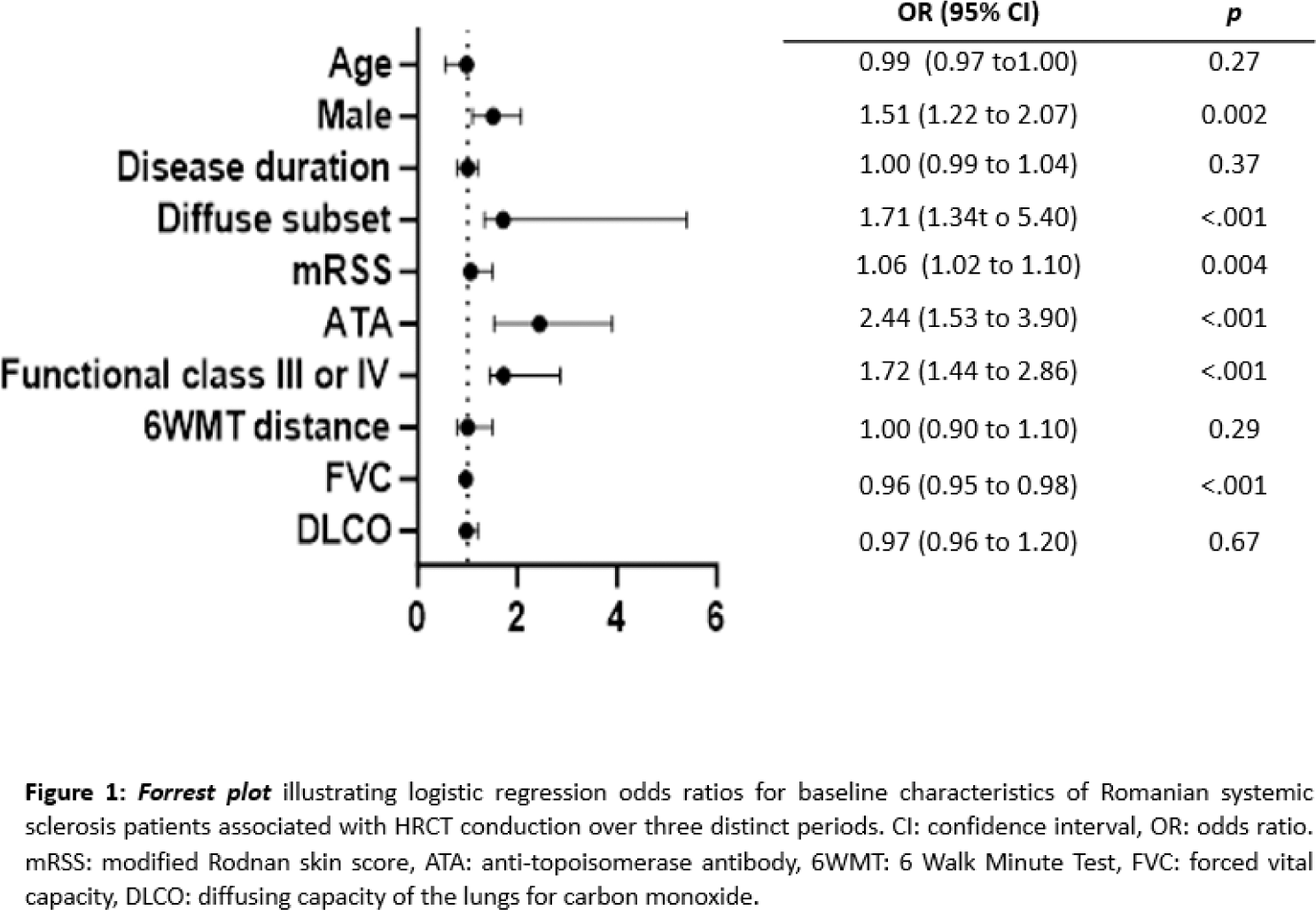
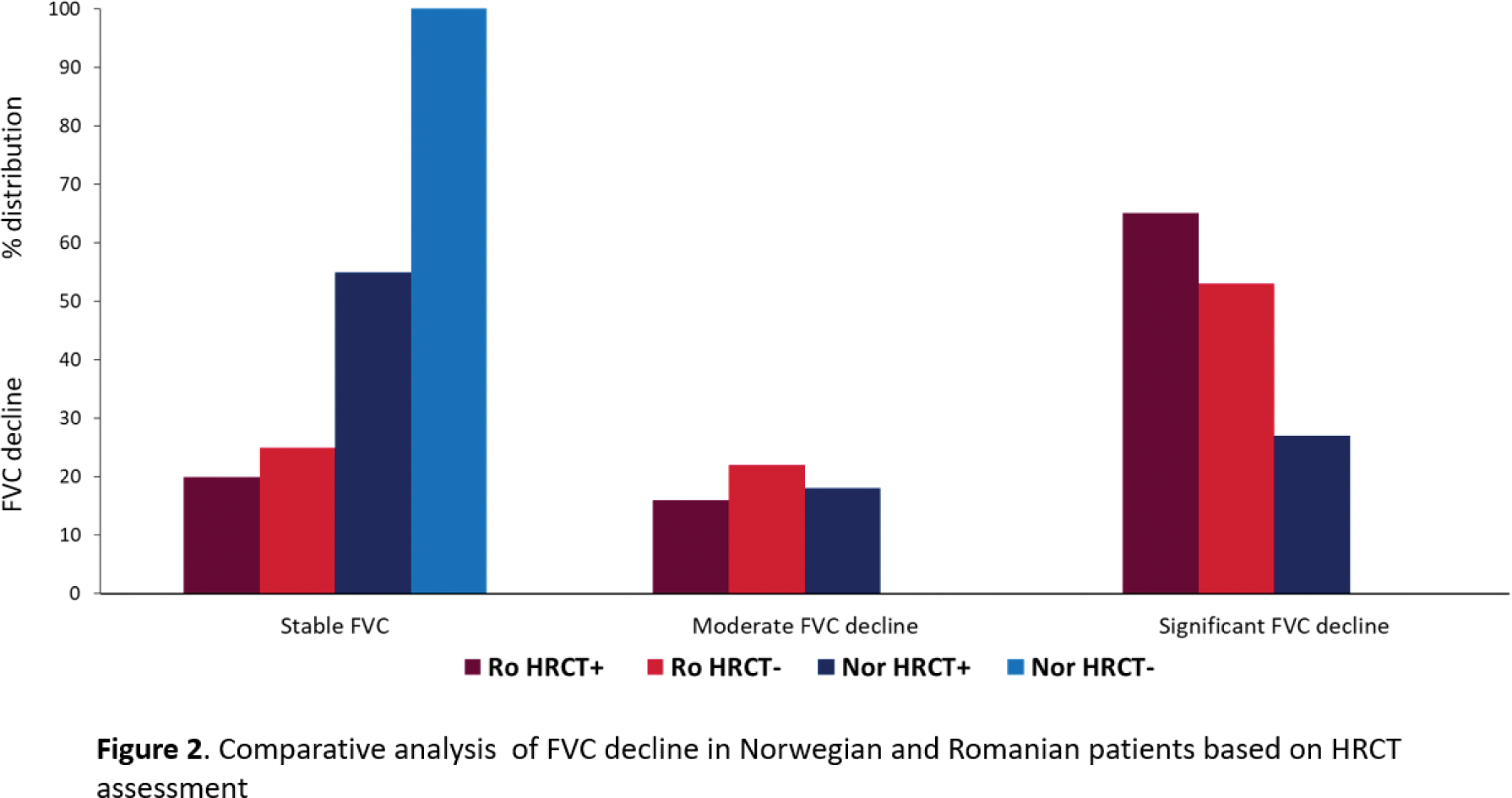
Results: Baseline screening for ILD with HRCT was performed in 37% (117) Ro- and 96% (871) Nor-SSc patients. The use of HRCT increased in the Ro-SSc cohort over time, with 60% of all patients undergoing HRCT after 2020, while remaining stable in the Nor-SSc cohort. HRCT screening in the Nor-SSc cohort was not based on risk factors for ILD. In the Ro-SSc cohort, HRCT screening was significantly associated with SSc-ILD risk factors, including male sex, diffuse cutaneous SSc, anti-topoisomerase I positivity, presence of respiratory symptoms, and lower FVC (Figure 1). Among all patients who underwent HRCT, 95% (190) of Ro- and 45% (388) of Nor-SSc were diagnosed with ILD. At the time of ILD diagnosis, Ro-SSc patients showed longer disease duration (4.41±3.2 vs. 2.1±2.9 years, p<.001) and more severe ILD with lower FVC (74.4±17.4% vs 90.4±23.7, p<001), and a higher prevalence of ≥20% fibrosis on HRCT (40% vs 11%, p<.001), compared to the Nor-SSc cohort. To estimate the impact of only screening SSc patients with risk factors for ILD, we next assessed patients in the Ro-SSc cohort not screened for ILD by HRCT and compared them to patients screened with HRCT and Nor-SSc patients without HRCT. Among the 40% of Ro-SSc cases without HRCT, a significant number experienced FVC decline, while Nor-SSc patients without HRCT showed no decline (Figure 2). The likelihood of ILD in Ro-SSc patients without HRCT was confirmed through logistic regression [OR 1.95 (0.49-2.63), p<0.001)]. In total, 23% of Ro-SSc and 30% of Nor-SSc patients died. As expected, in both HRCT-assessed SSc cohorts, FVC decline was predictive for mortality (Ro-SSc: HR 4.32, 95%CI 0.21-6.58, p<.001; Nor-SSc: HR 2.05, 95% CI 0.42-10.0, p<.001). In Ro-SSc patients without HRCT, FVC decline was predictive for mortality in the same range (HR 4.72, 95 % CI 1.43-15.5, p=0.01).
Conclusion: Our results suggest that upfront HRCT screening of all SSc patients allows for an earlier diagnosis of ILD. Selective HRCT referral practices based on known risk factors leave a significant number of progressive ILD cases undiagnosed, with missed opportunities for treatment. Overall, the current results suggest that referring all newly diagnosed SSc patients to primary ILD screening by HRCT is warranted.
REFERENCES NIL. Acknowledgements: NIL.
Disclosure of Interests: Cristina Nita: None declared, Laura Maria Groșeanu: None declared, Daniela Opriș-Belinski: None declared, Mihaela Popescu: None declared, Athir Eddan: None declared, Emily Langball: None declared, Håvard Fretheim Boehringer Ingelheim, Bayer, Henriette Didriksen: None declared, Hilde Jenssen Bjørkekjær Jannsen, Phuong Phuong Diep: None declared, Torhild Garen: None declared, Mike Durheim: None declared, Øyvind Midtvedt: None declared, Trond M Aaløkken: None declared, Oliver Distler Boehringer Ingelheim, Janssen, Medscape, CITUS AG, 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Orion, Prometheus, Redxpharma, Roivant, Topadur and UCB, Øyvind Molberg: None declared, Andra Bălănescu: None declared, Anna-Maria Hoffmann-Vold Boehringer Ingelheim, Boehringer Ingelheim, Janssen, Medscape, Merck Sharp & Dohme, Novartis and Roche, ARXX, BMS, Boehringer Ingelheim, Genentech, Janssen, Medscape, Merck Sharp & Dohme and Roche, Boehringer Ingelheim, Janssen.