

Background: Sarcoidosis is a heterogeneous multi-system disease in which therapueitic options are limited. Prednisolone has been the hallmark of treatment alongside introduction of methotrexate, mycophenolate and azathioprine. Infliximab although not licenced, remains the 3rd line therapy for sarcoidosis resistant to steroid and DMARDs.
Objectives: To determine the efficacy, safety and tolerability of intravenous infliximab therapy in sarcoidosis.
Methods: Patients who received infliximab therapy for a diagnosis of sarcoidosis between 2000 – 2023 were identified from the sarcoidosis database at King’s College Hospital. Data for ethnicity, age, gender, baseline organ involvement concurrent DMARD use, ACE and Il2Ra levels, and treatment response was recorded.
Results: In total 18 patients with sarcoidosis received infliximab, of which 75% were of Black ethnicity. Neurological involvement was most common at presentation (n=10). This was followed by pulmonary (n=9) and cutaneous (n=5). Other organ involvement detected by clinical examination or PET-CT included ocular (n=2) muscle (n=2), joint (n=7) liver (n=2%), splenic (n=2) breast (n=1) and aortic (n=1). Histological confirmation of granulomatous disease was made in all patients. Cutaneous manifestations at presentation included erythema nodosum (n=2) nodular eruptions (n=2) and ulcerating lesions (n=1). Serum ACE was measure in 10 patients at presentation; median 63UL (IQR 36-75) and soluble IL2R was measured in 8 patients, median 2219 ng/L (1719-5229). High resolution CT findings at presentation included nodules (n=7) consolidation (n=1) and ground glass and fibrosis (n=2), bronchiectasis (n=1and a pleural effusion (n=1). During their sarcoid work up, 2 patients were diagnosed with cardiac involvement: myocarditis (n=1) and pulmonary hypertension (n=1). Ocular involvement was detected by screening in 4 individuals: pan uveitis (n=2) and retinal vasculitis (n=2). All patients received prednisolone treatment (median dose 20g, IQR 20 to 30). At diagnosis over 1/3rd of the cohort (n=7) received csDMARD and 1 patient commenced infliximab as csDMARD therapy was contraindicated due to first trimester of pregnancy. Infliximab was commenced 27 months (IQR, 12 -128) after diagnosis. Indications included neurological (n=10) pulmonary (n=4), cutaneous (n=2) musculoskeletal (n=1) and breast (n=1) involvement. Of the cohort, 16 patients were prescribed prednisolone at the time of their first infliximab dose. Prednisolone tapering was achieved in 68% (11/16) and 38% (6/16) had managed to stop prednisolone completely. Sixteen patients responded to infliximab therapy, and all but one remain on treatment. This one individual achieved complete remission of cutaneous disease after 14 months of therapy. Two patients did not respond to infliximab; 1 failed to achieve remission after 6 months and has subsequently commenced tofacitinib, and the other did not tolerate infliximab and is under consideration for upadacitinib. There were no serious adverse events
Conclusion: In this cohort, infliximab was effective in disease refractory to csDMARDs. This included neurosarcoidosis, but also pulmonary, ocular and cutaneous involvement. Prednisolone was successfully tapered. Jak inhibitors may be promising as a second line agent in indivdiuals who do not response to anti-TNF therapy.
REFERENCES: NIL.
Serum Angiotensin Converting Enzyme (ACE) levels at baseline and after 3,6,12 months of Infliximab therapy.
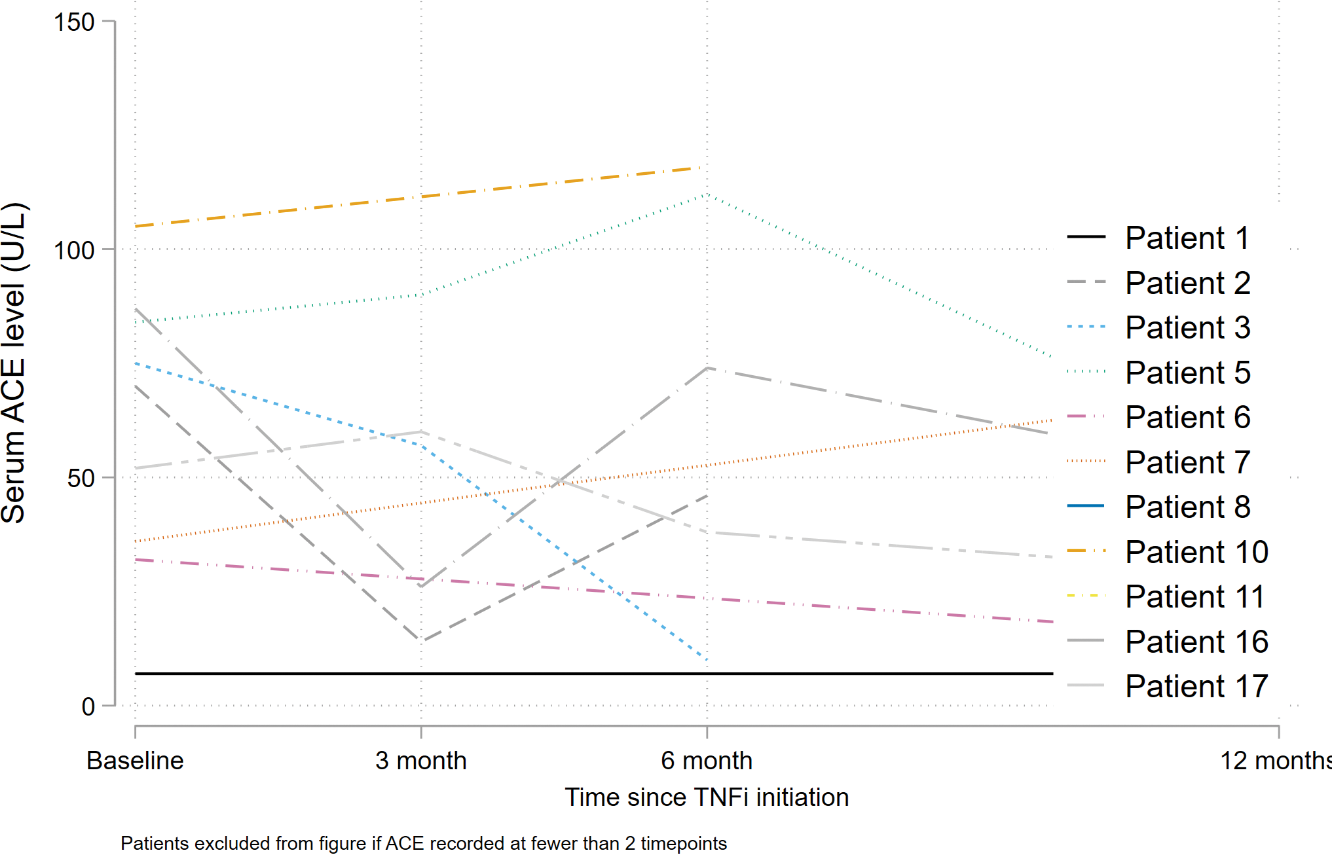
Acknowledgements: Centre for Rheumatic Disease, KCL.
Disclosure of Interests: Deepak Nagra Abbvie, Katie Bechman pfizer, Sayed Bukhari: None declared, Christopher baldwin: None declared, Edward Alveyn: None declared, Maryam A. Adas: None declared, Hemanth Kumar Molabanti: None declared, Komal Noorani: None declared, Kevin Tsoi: None declared, Mark Russell: None declared, James Galloway AbbVie, Biovitrum, BMS, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB.