

Background: The number of people living with HIV in North America was recently estimated at 980, 000, with a prevalence of 0.6%. The prevalence of sarcoidosis in the United States is 10.9/100.000 whites and 35.5/100.000 blacks. Despite these figures, HIV infection and sarcoidosis are rarely reported since two conditions are characterized by distinct immune responses. HIV infection is characterized by a profound depletion of Th1-type CD4 cells secreting components of the immune response involved in granuloma formation, namely IL-2 and INF-γ. On the other hand, sarcoidosis usually appears after a significant increase in CD4 cell count and a significant decrease in HIV load. Antiretroviral therapy (HAART) reduces the incidence of opportunistic infections in HIV-infected patients. However, various autoimmune diseases, including sarcoidosis may worsen at the initiation of HAART due to the recovery of Th1-type CD4 cell function. Paradoxical worsening of an existing infection or disease process soon after initiation of HAART is called immune reconstitution inflammatory syndrome (IRIS).
Objectives: Determine the risk of sarcoidosis in HIV patients, based on HAART usage, and analyze the demographic characteristics and diagnosis of IRIS of each group at baseline.
Methods: This is a nationwide, retrospective study. We used the global, multicenter research network (TrinetX) database from 83 large healthcare organizations across multiple countries. We included patients older than 18 years old with a diagnosis of HIV, for the last 10 years (between January 2013 and December 2023), using the International Classification of Disease-10 codes. We compared HIV patients on HAART with HIV patients not on HAART, using a 1:1 propensity score matching that accounted for demographics variables (age, gender, race), corticosteroid use and diagnosis of IRIS at baseline. Index event was defined as the initial appearance of the HIV diagnosis and corresponding medication status in the patient records. The outcome of interest was the incidence of sarcoidosis. Patients displaying this outcome before the index event were excluded from the analysis. The exposure for the outcome was anytime after the diagnosis of HIV during the time window. Odds ratios with 95% confidence intervals were calculated for each outcome. A two-sided p-value less than 0.05 was considered statistically significant.
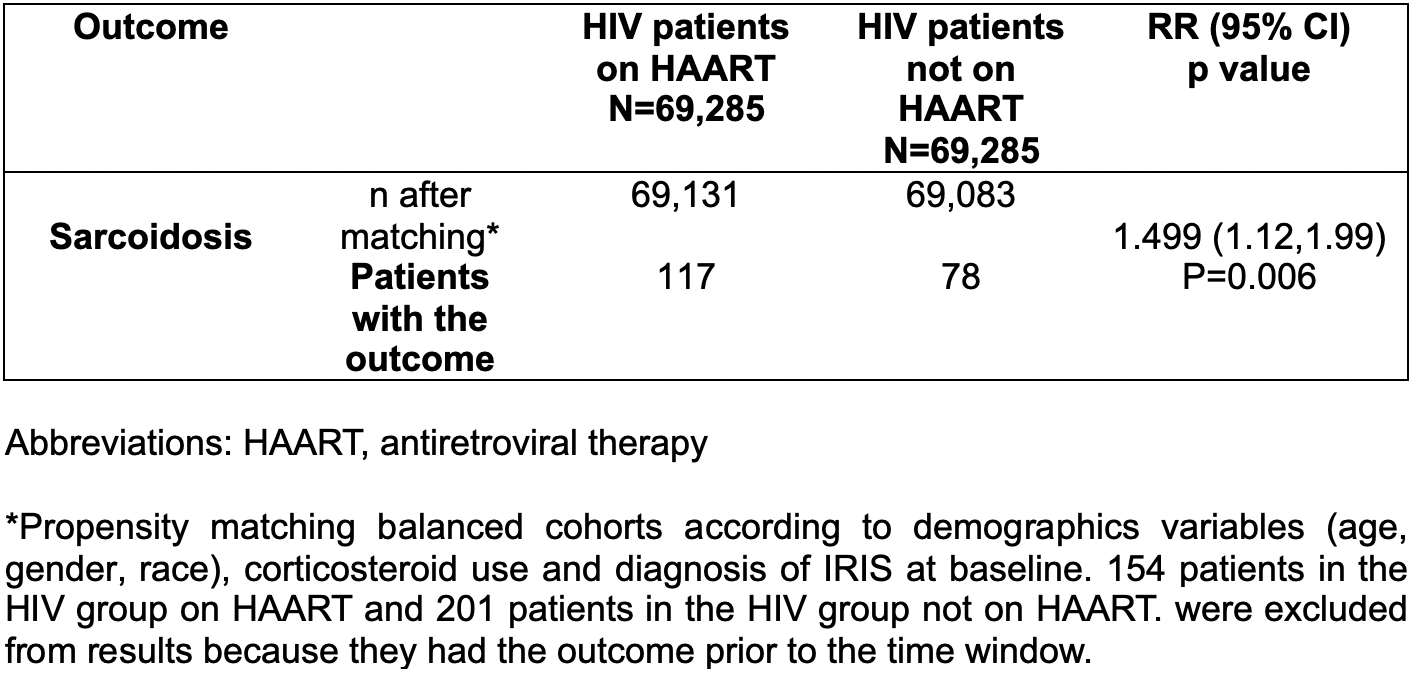
Results: We identified 142,086 HIV patients on HAART and 69,359 HIV patients not on HAART. At baseline, the mean age at diagnosis was 44.9 ± 13.5 and 45.2 ± 14.4 years for HIV patients on HAART and HIV patients not on HAART, respectively. Male gender was predominant in both groups (nearly 70% of the patients) and black or African American patients constitute the most common race in both subgroups (41.4 and 39.7 %, respectively). Similarly, diagnosis of IRIS and use of corticosteroids were more common among HIV patients on HAART (Table 1). After propensity score matching, patients who were taking HAART, had higher risk of developing sarcoidosis (RR= 1.499, CI95% 1.12,1.99; p=0.005) (Table 2) when compared with HIV patients not on HAART.
Conclusion: HIV patients receiving HAART have a higher risk of developing sarcoidosis than patients not receiving HAART. These findings encourage further investigation of the immune dynamics associated with HAART administration and its potential role in the pathogenesis of sarcoidosis. Because the prevalence of both diseases remains significant, understanding their complex relationships is critical to optimizing patient care and informing future treatment strategies.
Table 1. Baseline Patient Characteristics of Study groups, Before and After Propensity Score Matching
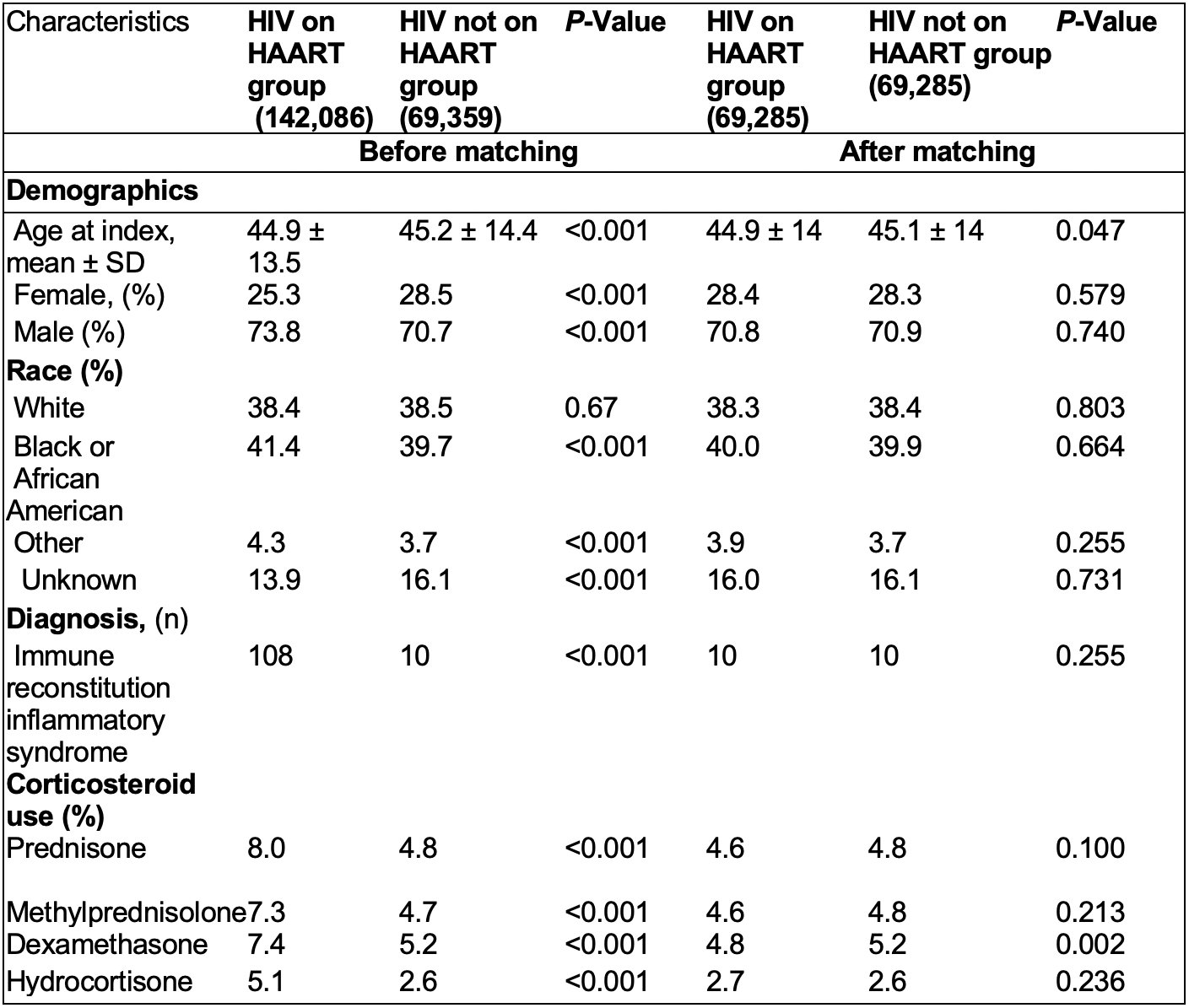
Table 2. Risk of sarcoidosis in HIV patients grouped by HAART usage.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.