

Background: The use of TNF inhibitors (TNFi) during pregnancy shows very reassuring evidence regarding adverse pregnancy outcomes. However, transplacental transfer of most TNFi occurs in the second half of pregnancy and may result in detectable drug levels of in-utero exposed infants. This raises concerns about a potential impact on neonatal immunity, including an increased risk of infections.
Objectives: To inform the EULAR task force on the safety of antirheumatic drugs during pregnancy by a systematic literature review (SLR). This SLR and meta-analysis focussed on the risk of serious infant infections (SII) after in-utero exposure to TNFi.
Methods: We searched Medline, Embase, Cochrane and LactMed from April 2015 to April 2023, including articles in English, French, Spanish and German. For outcomes such as SII, that were not included in the 2016 EULAR points to consider[1], the search was conducted from January 2000 onwards. All studies reporting SII in relation to maternal treatment with TNFi during pregnancy and further reporting of a control group (either diseased controls with no exposure to TNFi or healthy controls) were eligible for inclusion, independent of the underlying disease (including rheumatic diseases, but also autoimmune and autoinflammatory diseases). Studies reporting biologic treatment with >80% of patients receiving TNFi were also considered. SII were defined as any infection in the first year of life that required hospitalization. The meta-analysis comprised different approaches to estimate the risk of SII: (I) pooled prevalences for TNFi, diseased and healthy controls, (II) pooled unadjusted odds ratio (OR) for TNFi vs. diseased controls, (III) pooled risk of estimates adjusted for confounding factors for TNFi vs. diseased controls per risk estimate. Since SII can be considered as rare events, the different adjusted estimates were also combined into one pooled estimate. Studies with at least ten infants per treatment group were respected in the meta-analysis. The overall effects are based on a random effects model (Paule Mandel estimates or heterogeneity, Knapp Hartung method for confidence interval (CI) computation). If only two studies were available, a fixed effects model was applied. A risk of bias assessment was performed using the Newcastle-Ottawa scale.
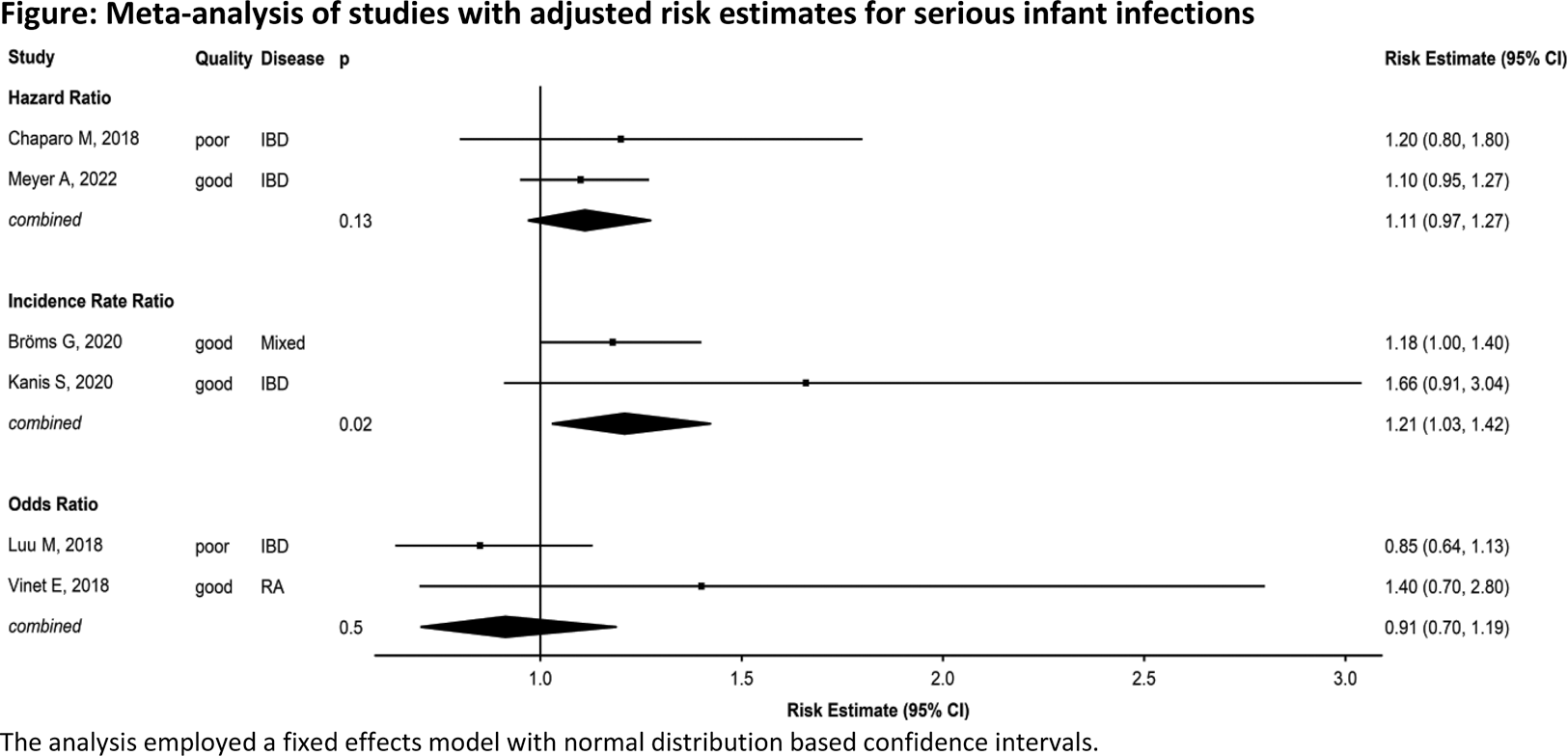
Results: Within the 307 identified publications (including all treatments and outcomes considered in SLR), 14 cohort studies were eligible for inclusion. Ten studies were of good quality, four of poor. The studies reported on a total of 7,826 infants with in-utero exposure to TNFi, 39,930 diseased controls and 646,011 healthy controls (Table 1). The majority of studies included infants of mothers with inflammatory bowel disease (n=11).
Pooled prevalences were 0.08 (95% CI 0.06, 0.12; 13 studies) for TNFi, 0.07 (0.05, 0.10; 12 studies) for diseased and 0.17 (1 study) for healthy controls. Pooled unadjusted OR of serious infections in infants with in-utero exposure to TNFi vs. diseased controls was 1.20 (95% CI 1.07, 1.34; 12 studies), pooled adjusted estimates ranged between 0.91 and 1.21 (6 studies, Figure 1). Pooling all adjusted estimates (aHR, aOR, aIRR) resulted in a risk estimate of 1.12 (95% CI 0.94, 1.32). Furthermore, four studies investigated TNFi exposure in the third trimester, leading to a pooled risk estimate of 0.94 (95% CI 0.62, 1.43).
Conclusion: After in-utero exposure to TNFi, SII occurred in 8% of infants compared to 7% of infants whose mothers had the same disease, but were not exposed to TNFi during pregnancy. Even though the unadjusted pooled OR showed a slight risk increase of 20%, this result should be interpreted with caution since potential risk factors such as preterm birth or other complications were not accounted for. Of note, the adjusted models did not show a signal for an increased risk of serious infections in infants associated with TNFi exposure during pregnancy.
REFERENCES: [1] Gotestam Skorpen, 2016, DOI: 10.1136/annrheumdis-2015-208840.
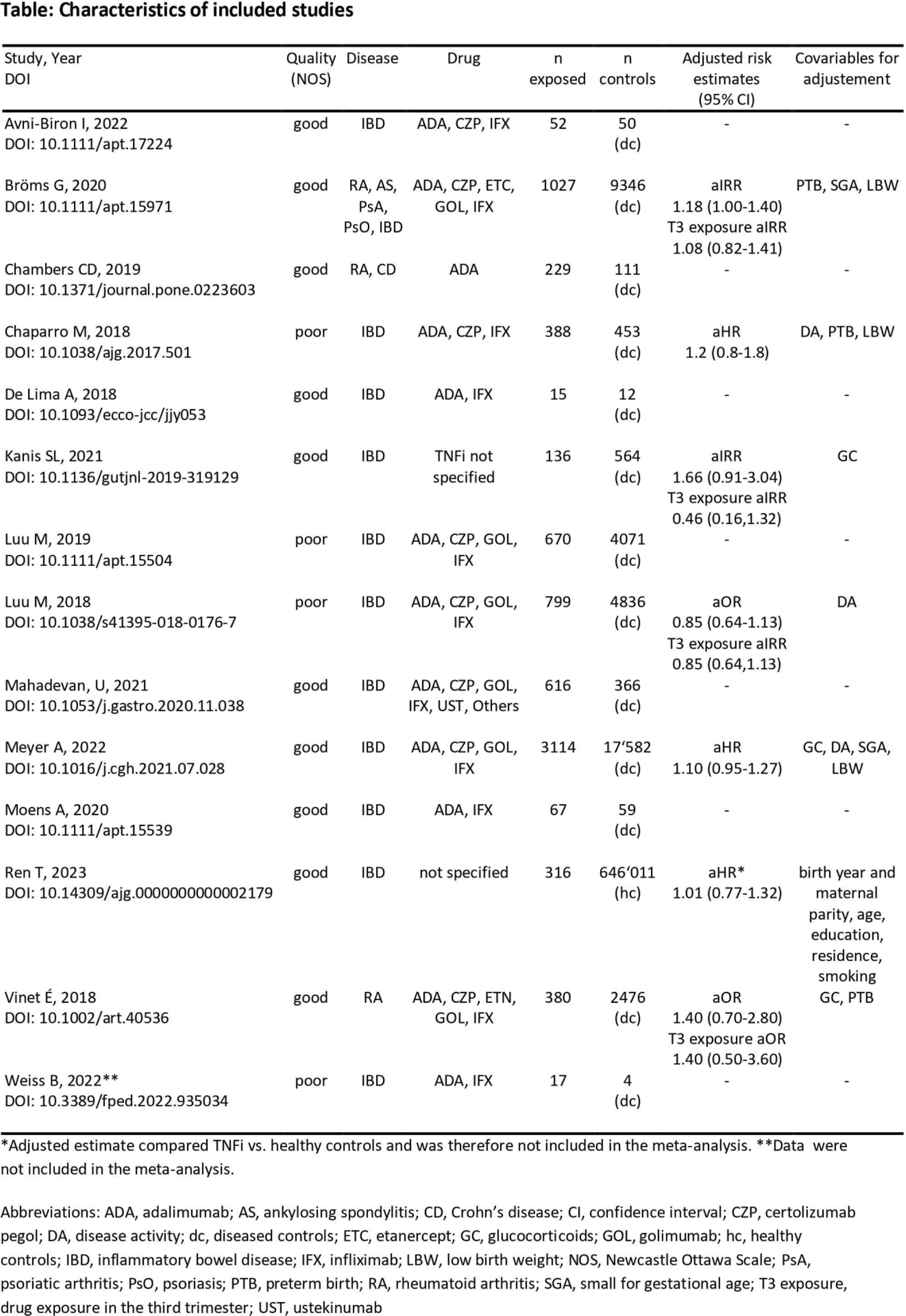
Meta-analysis of studies with adjusted risk estimates for serious infant infections
The analysis employed a fixed effects model with normal distribution based confidence intervals.
Acknowledgements: NIL.
Disclosure of Interests: Linda Rüegg: None declared, Sabrina Hamroun: None declared, Andrea Pluma Sanjurjo: None declared, Malte Kramer Bayer, Axel Finckh: None declared, Frauke Förger Speaker’s bureau: Mepha, Roche, UCB Pharma, MSD, Grant/research support from: UCB Pharma, GSK, Yvette Meissner Speakers Bureau: Lilly und Pfizer.