

Background: Approximately 50% of women of childbearing age with autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis (PsA), plaque psoriasis, Crohn’s disease and axial spondylarthritis (axSpA) require therapeutic intervention.[1] However, there are limited data on the pharmacokinetics (PK) of treatments during pregnancy. Certolizumab pegol (CZP), a PEGylated Fc-free tumour necrosis factor inhibitor, may be used in adults for the treatment of the aforementioned autoimmune diseases. We report PK and safety results from the first longitudinal assessment of CZP PK during pregnancy.
Objectives: To investigate the longitudinal PK and safety of the use of CZP during pregnancy and post-partum by measuring plasma CZP concentration and monitoring treatment-emergent adverse events (TEAEs).
Methods: The CHERISH study (NCT04163016) was a multicentre, longitudinal, interventional, prospective, open-label phase 1B exploratory study evaluating the impact of pregnancy on the PK of CZP. The study consisted of a screening period, pregnancy period (up to 40 weeks), post-partum period (up to 13 weeks), and a safety follow-up contact (five weeks after final study visit). Patients (pts) were pregnant (≤10 weeks gestation at time of enrolment) and on stable, maintenance dose CZP treatment for ≥12 weeks prior to being enrolled in the study. Predose PK samples were collected ≤4 hours prior to dosing every 4 weeks (Q4W) starting with the first dose after enrolment. Postdose PK samples were taken 7 days after CZP dose administration every 8 weeks. One predose sample and one postdose sample were taken 11–14 weeks post-partum.
The primary variable was predose and postdose plasma CZP concentrations adjusted for disease phenotype, gestational week, trimester and pre/postdose, assessed in pts receiving ≥1 dose of CZP after enrolment (pharmacokinetic per protocol set); safety was assessed in pts receiving CZP at screening (safety set). Adjusted mean differences and 95% confidence intervals were estimated for overall plasma CZP concentrations during pregnancy vs post-partum using a linear mixed effects model.
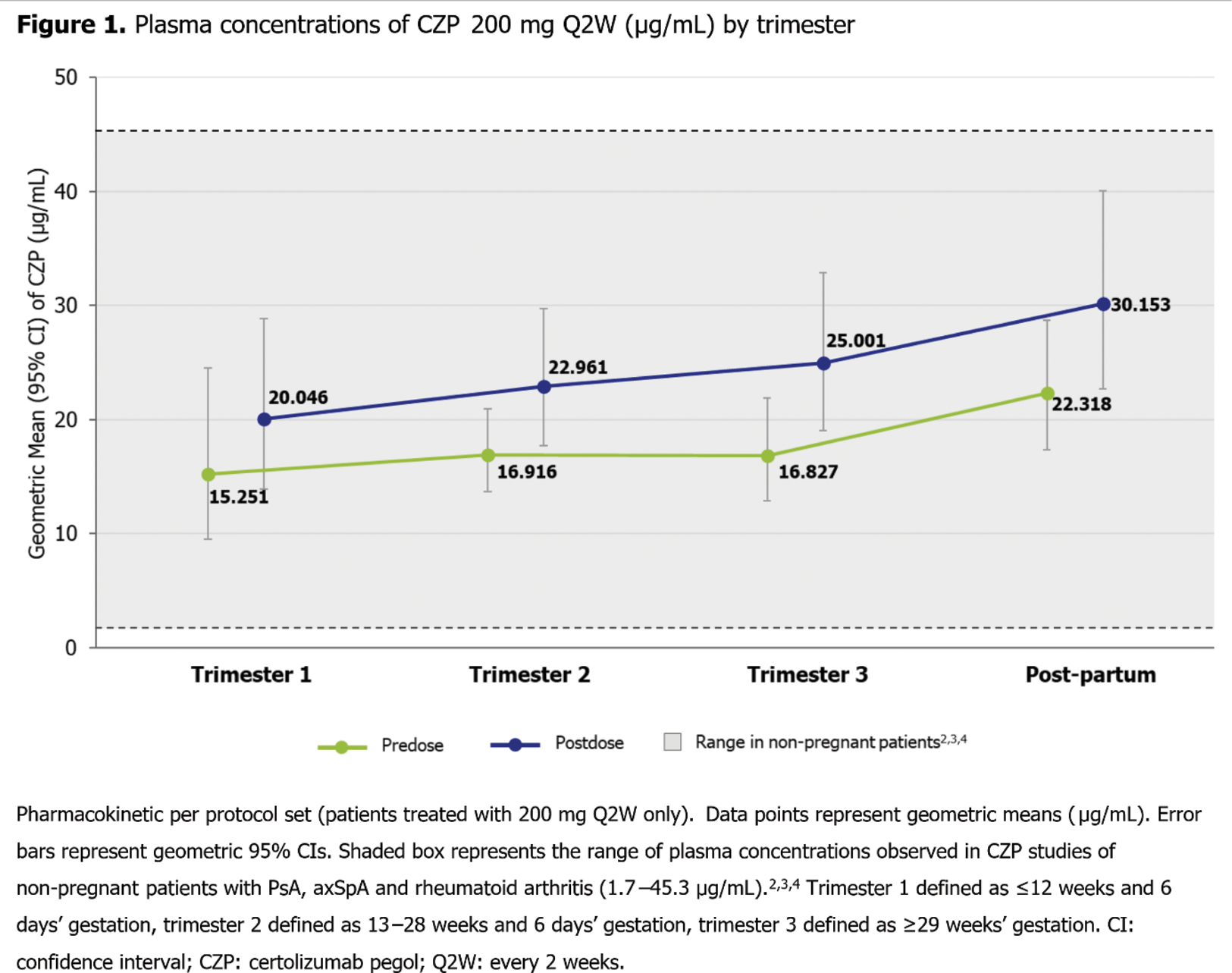
Results: Of the 21 enrolled pts (CZP 200 mg every two weeks [Q2W], n=15; CZP 400 mg Q2W, n=1; CZP 400 mg Q4W, n=5), 16 (76.2%) completed the study. The mean age of pts was 32.2 (standard deviation: 4.6) years and the most common primary indication for CZP treatment reported was rheumatoid arthritis (n=9 [42.9%]). The range of plasma CZP concentrations observed (Figure 1) was within the range of plasma concentrations observed in CZP studies of non-pregnant pts with PsA, axSpA and rheumatoid arthritis (1.7–45.3 μg/mL). 2,3,4
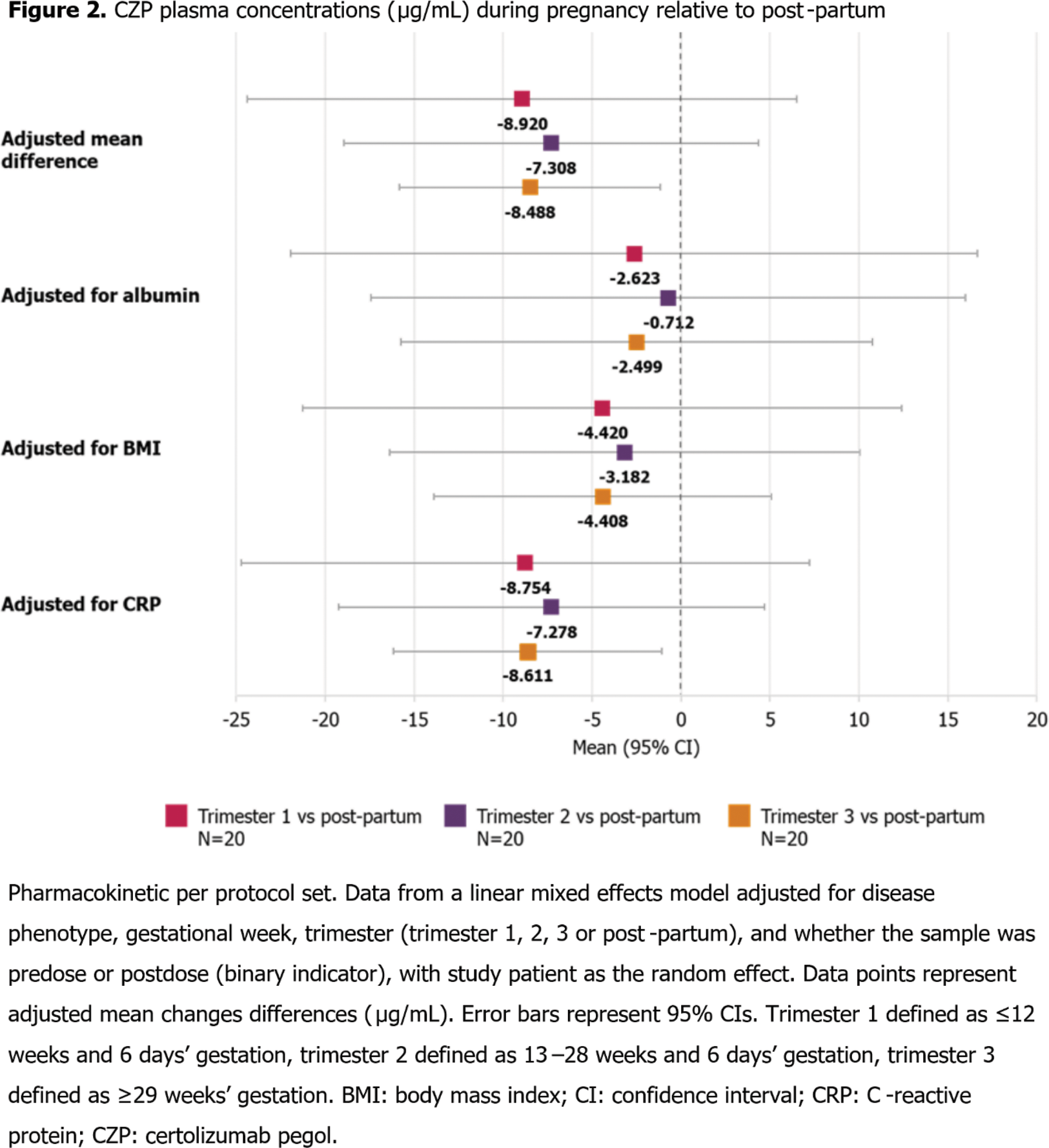
Plasma CZP concentrations were lower during pregnancy relative to post-partum, though between trimesters there were no large variations in in CZP concentrations. Mean differences in overall plasma CZP concentrations during pregnancy vs post-partum were reduced when accounting for individual serum albumin and body mass index, but not C-reactive protein (Figure 2).
The safety profile observed in this study was consistent with the known safety profile of CZP. 5 17 (81.0%) pts had a TEAE; the most common maternal TEAE was infections and infestations (n=12 [57.1%]). No study pt deaths, infant illnesses, or other safety concerns were reported.
Conclusion: PK data of CZP in pregnant pts were similar to non-pregnant pts and remained consistent throughout pregnancy, though pregnant pts had lower CZP plasma concentrations than post-partum. 2,3,4 This suggests that pts may remain on their dosing regimen during pregnancy, particularly as CZP has been shown to minimally cross the placental barrier, 1 to ensure stable plasma concentration throughout pregnancy.
REFERENCES: [1] Mariette X et al, Ann Rheum Dis 2017;77:228–33. 2. European Medicines Agency, Cimzia Assessment Report 2013; 3. Gehin JE et al, Arthritis Res Ther 2019;21:256. 4. Paul S et al, Clin Transl Sci 2020;13(4):743–51. 5. Curtis JR et al, RMD Open 2019;5(1):e000942.
Acknowledgements: Funded by UCB Pharma. Medical writing support provided by Costello Medical and funded by UCB Pharma.
Disclosure of Interests: Megan E.B. Clowse UCB Pharma, GSK, Grant/research support from: UCB Pharma, GSK, Radboud J.E.M. Dolhain Speaking fees from UCB Pharma, Roche, Abbvie, Genzyme, Novartis, AstraZeneca, and Eli Lilly., Unrestricted research grants from Dutch Arthritis Association, ZonMw, UCB Pharma and Galapagos., Stephanie Finzel Speaker’s honoraria: Abbvie, Chugai, Galapagos, GSK, Novartis, UCB Pharma, Consultant for: Novartis, NovoNordisk, Grant/research support from: Novartis, Frauke Förger Speakers bureau from Mepha, Roche, UCB Pharma, MSD., Grant/research support from UCB Pharma., Cornelia Glaser Speaker’s honoraria from Galapagos, GSK, Pfizer and UCB Pharma., Andrea Pluma Sanjurjo: None declared, Laura Shaughnessy Employee of UCB Pharma., Jagdev Sidhu Employee of UCB Pharma., Jemma Greenin Employee of UCB Pharma., Kathy Rice Employee of UCB Pharma., Marie Teil Employee of UCB Pharma.