

Background: Immunomodulatory agents (IA) are widely used for the treatment of inflammatory rheumatic diseases (IRDs). IA are known to be associated with small increase in infection risk and it is unclear what should be done in case of an intercurrent infection. Most guidelines and Summaries of Product Characteristics recommend temporary interruption, but based on indirect evidence, continuation of IA might be an equally safe option and perhaps even improve outcome of infections.
Objectives: To assess the effect of continuation of IA versus temporary interruption in case of an infection.
Methods: This study was a large, multi-centre, open-label, explorative randomized controlled strategy study among IRD patients using IA in the Netherlands. Patients were eligible if they had a clinical diagnosis of rheumatoid arthritis, psoriatic arthritis or spondylarthritis, were 16 years or older, used one or more IA, were able to read and communicate in Dutch and were not experiencing a clinical infection at time of inclusion (based on check in electronic health record and as reported by the patient). Participants were randomized 1:1 into either the intervention (continuation of IA during an infection) or control (temporary interruption of IA during an infection) group, stratified for the use of TNF inhibitors, oral glucocorticoids and risk of severe COVID-19 (based on the categorization of the CDC). Participants were instructed to either continue or temporary (until the infection was resolved) interrupt their IA (according to allocation) in case of their first clinically relevant infection, all in addition to standard care. A clinically relevant infection was defined as ≥ grade 2 according to the Common Toxicity Criteria for Adverse Events version 5.0 (CTCAE v5.0); i.e. needing oral treatment and/or limited instrumental activities of daily living (iADL). The primary outcome was the proportion of patients with a serious infection (defined as ≥ grade 3 according to the CTCAE v5.0), i.e. resulting in hospitalisation, administration of intravenous medication or death), analysed in the intention-to-treat (ITT) population using the Cochran-Mantel-Haenszel risk difference and confidence interval, stratified by the stratification factors use of TNF inhibitors, oral glucocorticoids and risk of severe COVID-19 (based on the categorization of the CDC). The ITT population consisted of all patients who experienced at least one clinically relevant infection. This study is registered in the Dutch Trial Register (ID: NL8922).
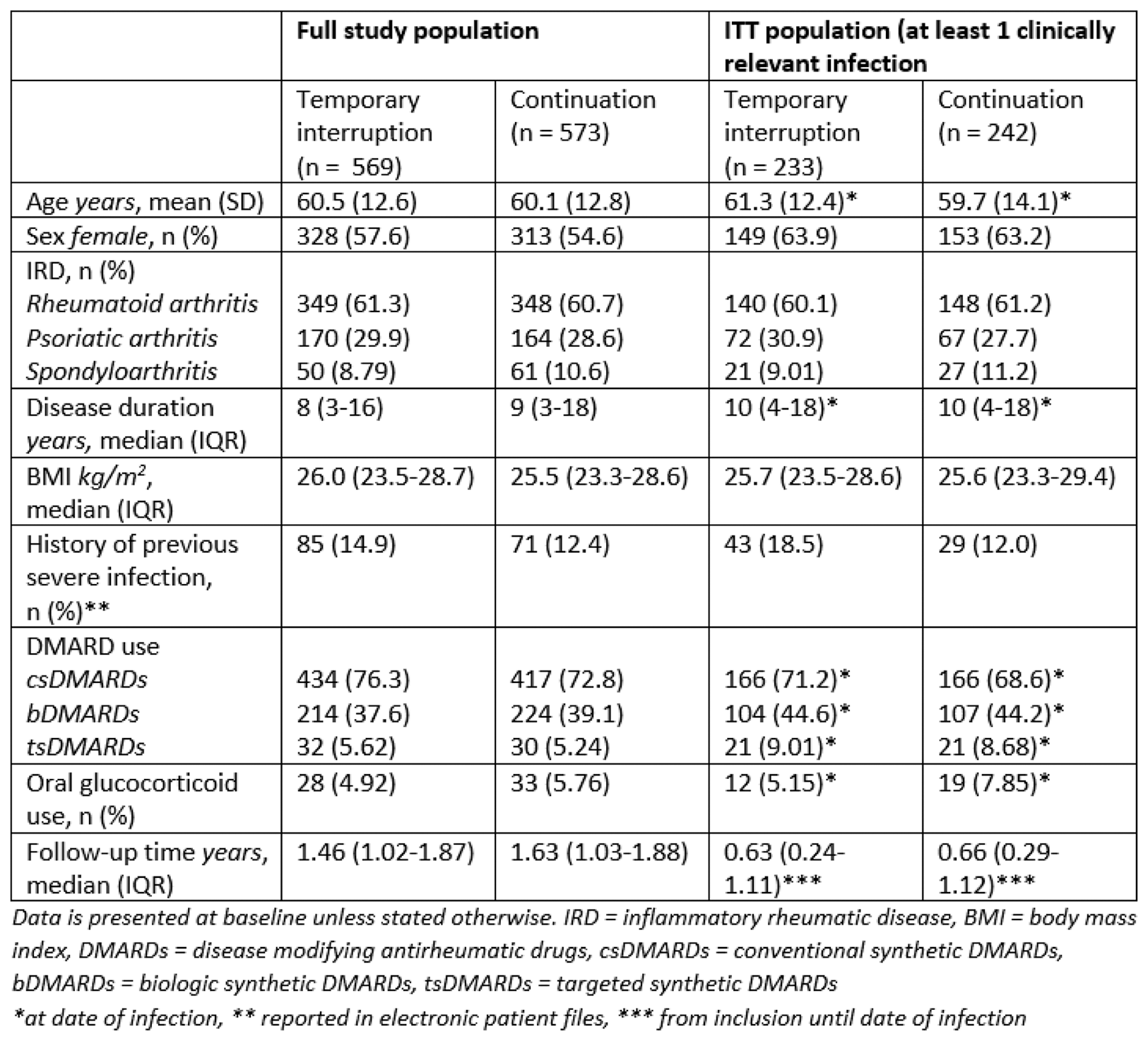
Results: In total, 1142 patients were included with a total follow-up time of 1667 patient years. 475 patients experienced at least one clinically relevant infection and were included in the ITT population. Table 1 shows patient, treatment and disease characteristics for the entire study population and the ITT population. Serious infections occurred in 12 out of 233 patients (5.15%) in the temporary interruption group and in 9 out of 242 patients (3.72%) in the continuation group, with an adjusted risk difference of 1.72% (95% CI -1.96 to 5.41) (figure 1).
Conclusion: Temporary interruption or continuation of IA resulted in similar outcome of infections. These findings suggest that continuation of IA is safe, although precision was somewhat limited due to low event rates. Secondary analyses will assess the effect of treatment received, taking into account non-compliance to the allocation, and investigate infection outcomes for different types of IA.
REFERENCES: NIL.
Table 1. Patient, disease and treatment characteristics
Flow diagram from included patients to patients with serious infection
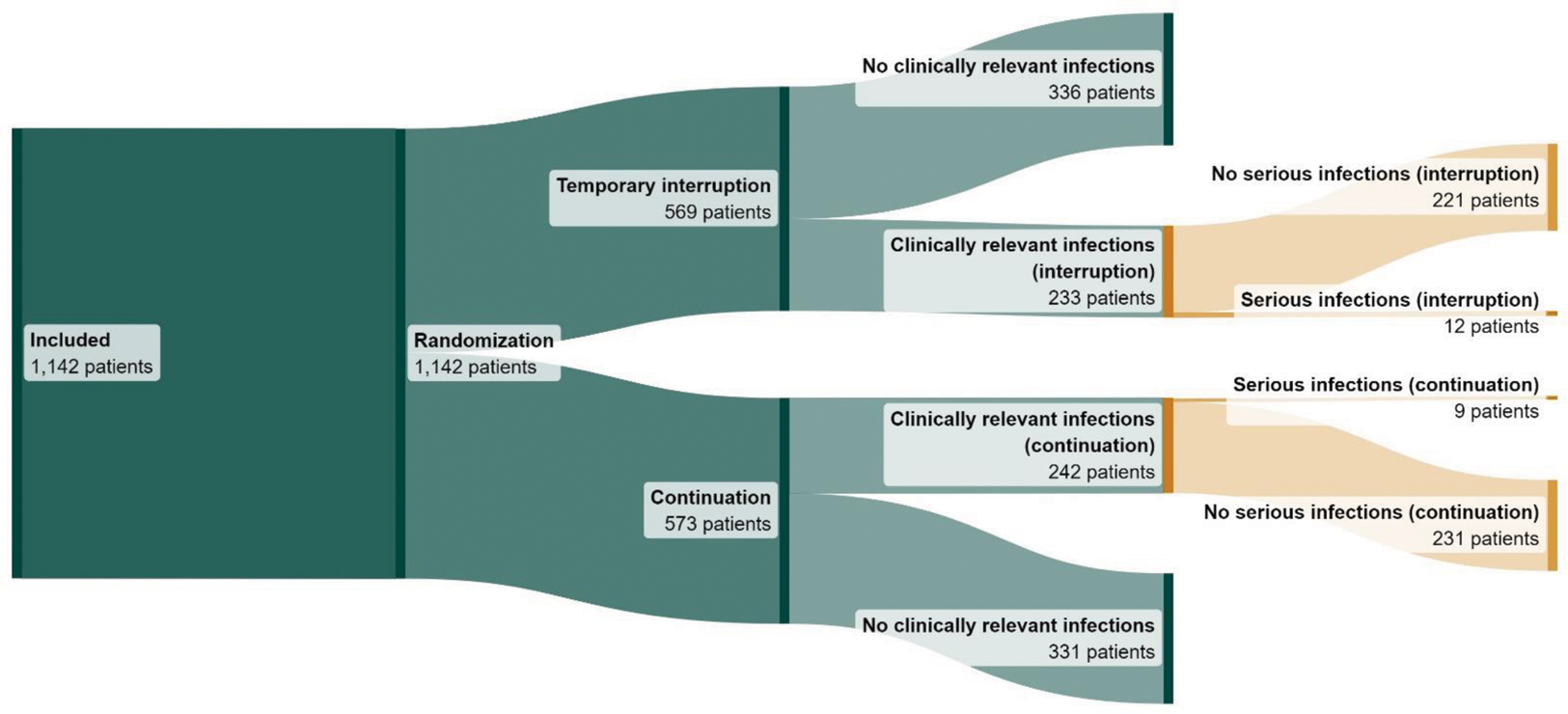
Clinically relevant infections are infections requiring oral therapy (e.g. antibiotic or antiviral therapy) or infections resulting in limitations in instrumental activities of daily living. Serious infections are infections requiring hospitalisation or intravenous administration of medication.
This flow diagram was made using SankeyMATIC.com
Acknowledgements: We would like to thank Lisa Schapink and Léon Raymakers for their support in the development of the trial study protocol.
Disclosure of Interests: Merel Opdam: None declared, Nathan den Broeder: None declared, Reinout van Crevel: None declared, Jasper Broen: None declared, Lise M. Verhoef: None declared, A.A. den Broeder Grants for research and quality of care projects to the institution from Lilly, Abbvie, Galapagos, Novartis, Pfizer, Gilead, Sanofi, Biogen, Celltrion.