

Background: Connective tissue disease-associated interstitial lung disease (CTD-ILD) presents a complex clinical scenario, often necessitating intensive therapeutic interventions such as cyclophosphamide pulse therapy. Despite its efficacy, the associated risk of infection remains a critical concern, prompting the need for a nuanced clinical predictive model.
Objectives: The primary objective of this study is to construct a robust clinical predictive model that can effectively discern the infection risk in CTD-ILD patients receiving cyclophosphamide pulse therapy. Such a model holds promise for optimizing patient care and treatment outcomes.
Methods: A retrospective analysis was conducted on 111 patients diagnosed with CTD-ILD who underwent cyclophosphamide pulse therapy. Comprehensive patient data, including demographic parameters, disease-specific characteristics, and treatment histories, were systematically collected. The analysis employed multivariate statistical methods to identify independent predictors of infection risk, leading to the development of a clinical predictive model.
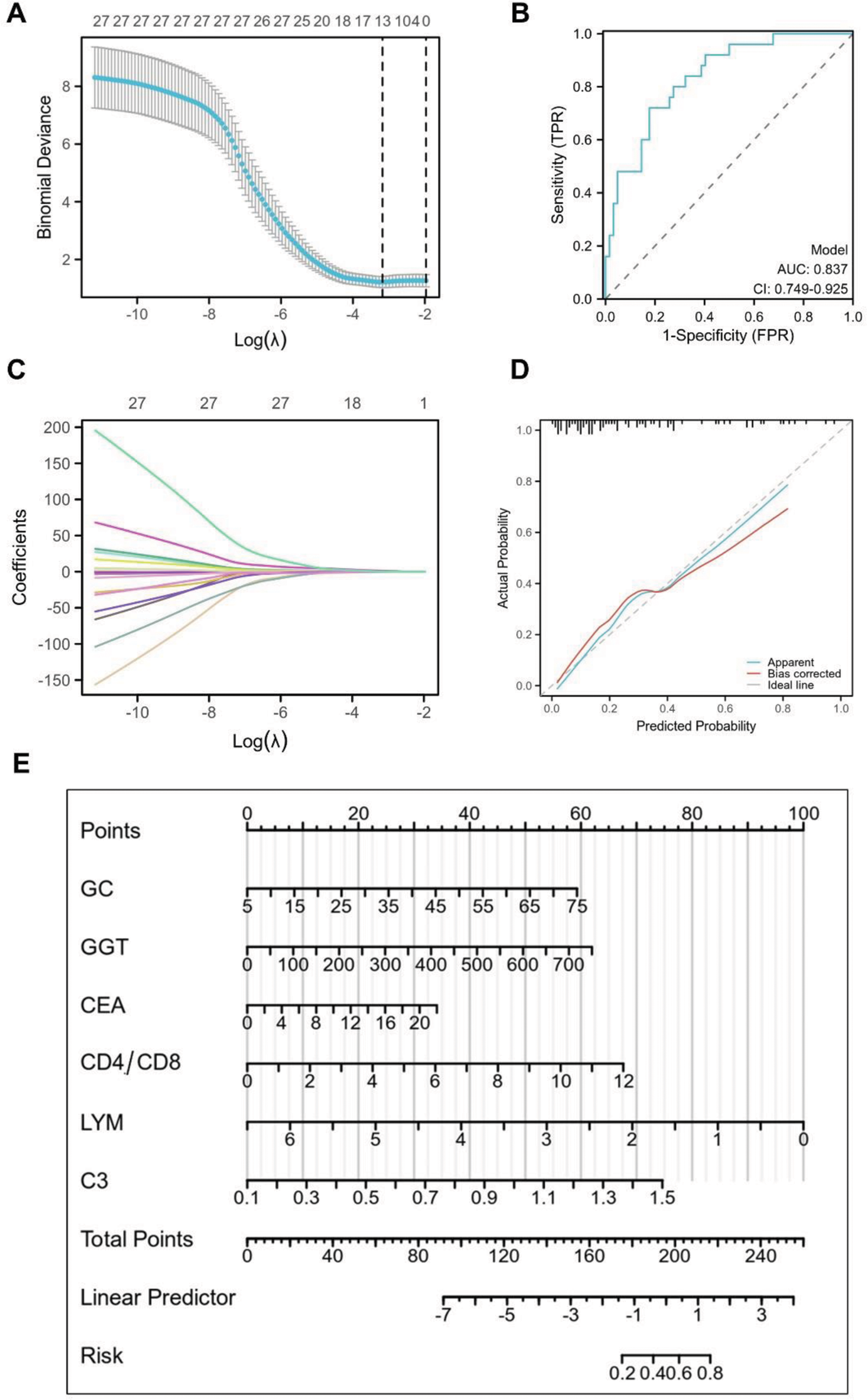
Results: A cohort of 111 confirmed CTD-ILD patients (mean age: 53.2±10.9 years; 72.1% female) was analyzed. During cyclophosphamide pulse therapy, 32 patients (28.8%) experienced infections, primarily concentrated in the initial three treatment cycles. The final model incorporated 6 key indicators (Oral glucocorticoids, GGT, carcinoembryonic antigen, CD4/CD8, lymphocyte, and C3), effectively discriminating between patients with and without infection events. The area under the receiver operating characteristic curve (AUC) for the model was 0.837 [95% confidence interval (CI) 0.749-0.925], indicating its high accuracy.
Conclusion: This study successfully developed a predictive model for infection risk during cyclophosphamide pulse therapy in CTD-ILD patients. The model, comprising 6 significant indicators, demonstrated strong discriminatory capabilities, with an AUC of 0.837. Implementation of this model in clinical practice may enhance the identification of patients at higher risk of infection during treatment, allowing for timely intervention and improved patient management. Further validation studies are recommended to confirm the generalizability and reliability of the model across diverse patient populations.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.