

Background: Upadacitinib (UPA), an oral JAK inhibitor, has demonstrated efficacy and safety across several rheumatic diseases, including RA, PsA, AS (also called r-axSpA), and nr-axSpA. Safety observed with JAK inhibition to date has highlighted the need to further characterize the long-term safety profile of individual JAK inhibitors across diverse patient populations.
Objectives: Describe the long-term integrated safety profile of UPA 15 mg across indications in rheumatology, in the context of active comparators, from the SELECT clinical program.
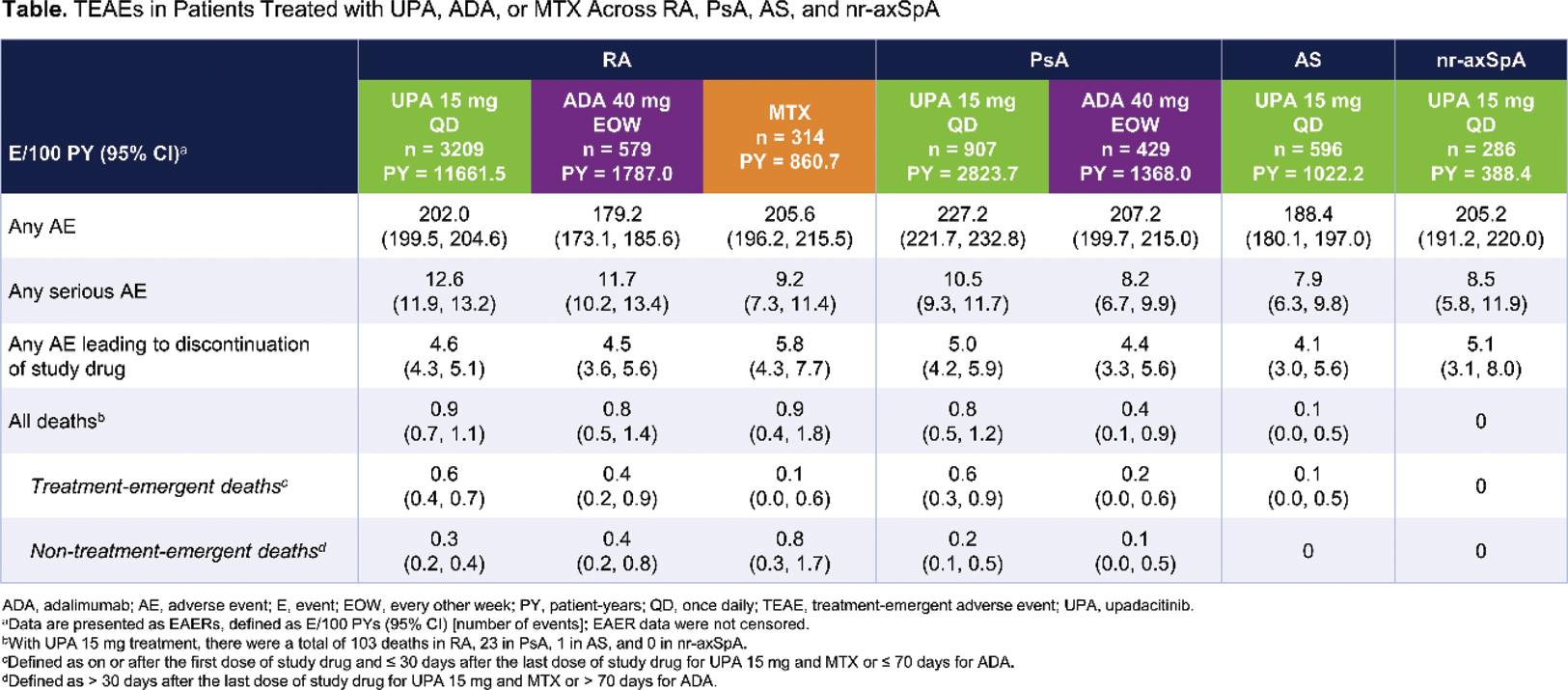
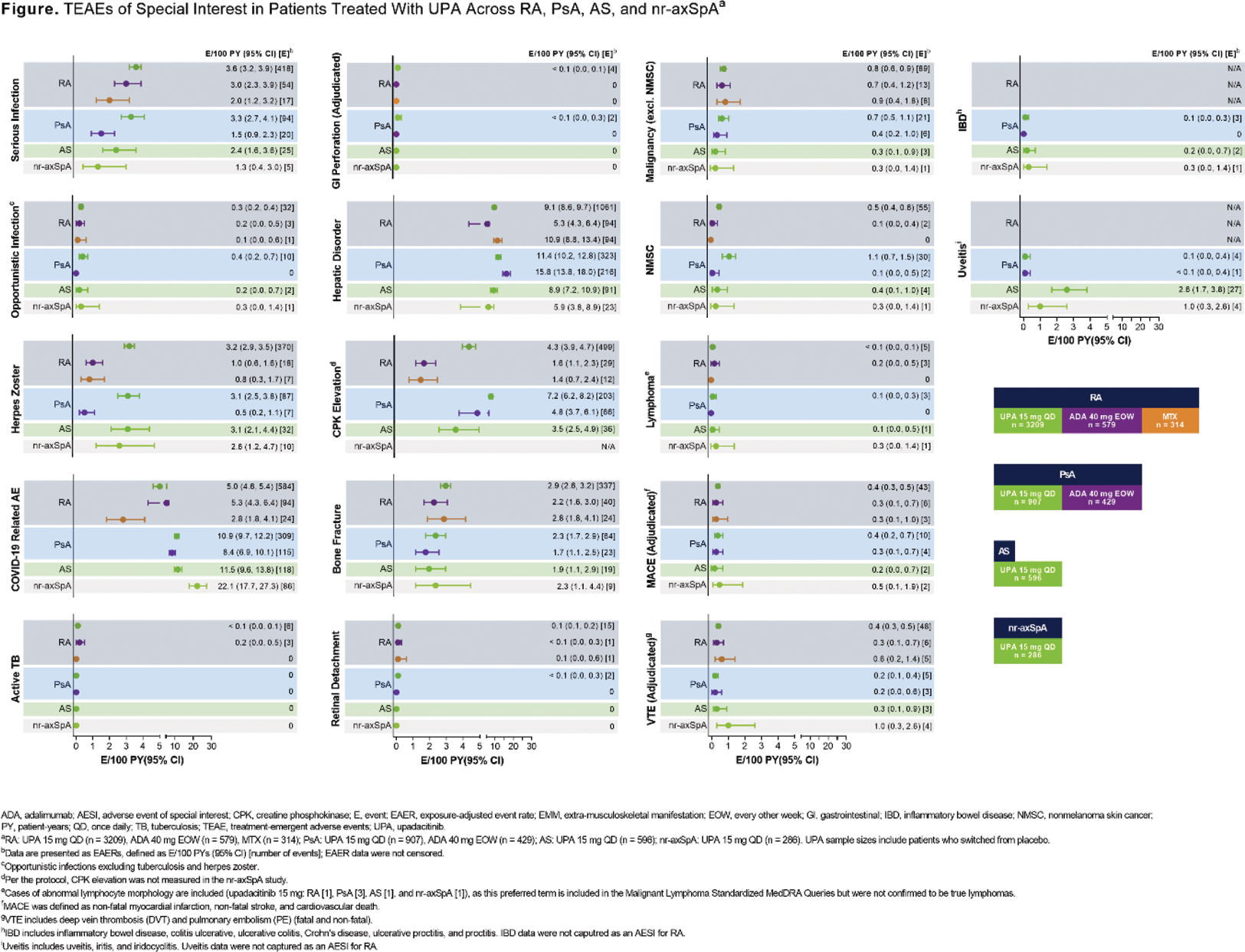
Methods: Safety data (cutoff date: 15 August 2023) from 11 phase 3 UPA trials were compiled for RA (6), PsA (2), AS (2; one phase 2/3), and nr-axSpA (1) for this analysis.[1,2] Treatment-emergent adverse events (TEAEs) with an onset on or after the first dose of study drug and ≤ 30 days after the last dose of study drug for UPA 15 mg and MTX, or ≤ 70 days for adalimumab (ADA), were coded using MedDRA version 26.0. TEAEs were summarized for RA (pooled UPA 15 mg, ADA, and MTX [1 study only for ADA and MTX]), PsA (pooled UPA 15 mg and ADA [1 study only]), AS (pooled UPA 15 mg), and nr-axSpA (UPA 15 mg). TEAEs are presented as exposure-adjusted event rates (EAERs; events/100 patient-years [E/100 PY] with 95% confidence intervals). Deaths, cardiovascular events, VTEs, and gastrointestinal perforations were adjudicated by blinded, independent committees using pre-specified definitions.
Results: In total, 4998 patients (RA, n = 3209; PsA, n = 907; AS, n = 596; nr-axSpA, n = 286) received ≥ 1 dose of UPA 15 mg, totaling 15,895.8 PYs of exposure, with the majority of exposure from RA studies (Table 1). The rate of adverse events (AEs) leading to discontinuation of study drug was generally similar across treatment groups (UPA 15 mg, ADA, and MTX) and diseases (RA, PsA, AS, and nr-axSpA). AEs leading to discontinuation of UPA 15 mg treatment varied widely; most frequently reported were pneumonia (RA; n=22/459), COVID-19 (PsA; n=7/121), headache (AS; n=3/42), and worsening axial spondyloarthritis and pulmonary embolism (nr-axSpA; both n=2/20). Rates of serious infection and opportunistic infection were generally similar across treatment groups and diseases; however, the rate of serious infection was higher with UPA 15 mg versus ADA in PsA (Figure 1). COVID-19 pneumonia was both the most common serious infection and serious AE with UPA 15 mg treatment for all diseases. Herpes zoster and elevated creatinine phosphokinase (CPK) were reported more often with UPA 15 mg versus active comparators in RA and PsA, with UPA 15 mg showing similarly elevated rates of herpes zoster across all diseases. Rates of malignancy excluding nonmelanoma skin cancer (NMSC) were generally similar across treatment groups and diseases. Higher rates of NMSC were observed with UPA 15 mg versus active comparators in RA and PsA. Similar rates of MACE and VTE were observed across treatment groups and diseases. Rates of extra-musculoskeletal manifestations (EMMs), including uveitis and inflammatory bowel disease, were generally low across PsA, AS, and nr-axSpA with UPA 15 mg treatment, with the highest number of events reported for uveitis in AS. The most common cause of death across all diseases was COVID-19/COVID-19 pneumonia. Real-world data, especially for diseases with more limited clinical trial exposure (ie, AS and nr-axSpA), are needed to further contextualize and confirm these findings.
Conclusion: With the exception of serious infection (in PsA), herpes zoster, elevated CPK, and NMSC, the rates of TEAEs were generally similar between UPA 15 mg and active comparators in RA and PsA. Across RA, PsA, AS, and nr-axSpA, UPA 15 mg demonstrated a generally consistent safety profile, with no new safety risks identified with long-term treatment, as shown in previous reports.[1,3,4]
REFERENCES: [1] Burmester G, et al. RMD Open. 2023;9:e002735.
[2] Deodhar A, et al. Lancet. 2022;400:369-79.
[3] Cohen SB, et al. Ann Rheum Dis. 2020;80:304-11.
[4] Burmester GR, et al. Rheumatol Ther. 2022;9:521-39.
Acknowledgements: AbbVie and the authors thank the patients, study sites, and investigators who participated in these clinical trials (NCT02706873, NCT02675426, NCT02629159, NCT02706951, NCT02706847, NCT03086343, NCT03104400, NCT03104374, NCT03178487, and NCT04169373). AbbVie funded these studies and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Monica R.P. Elmore, PhD of AbbVie. Editorial support was provided by Angela T. Hadsell of AbbVie.
Disclosure of Interests: Gerd R. Burmester AbbVie, Eli Lilly, Galapagos, Janssen, MSD, Pfizer, Roche, and UCB, AbbVie, Eli Lilly, Galapagos, Janssen, MSD, Pfizer, Roche, and UCB, Stanley B. Cohen AbbVie, Amgen, Boehringer Ingelheim, Gilead, Pfizer, Roche, and Sandoz, AbbVie, Amgen, Boehringer Ingelheim, Gilead, Pfizer, Roche, and Sandoz, Atul Deodhar Novartis and Pfizer, AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB Pharma, AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB Pharma, Eduardo Mysler AbbVie, Alpine Immunology, AstraZeneca, BMS, Eli Lilly, GSK, Hi Bio, Janssen, Novartis, Pfizer, Roche, and Sandoz, AbbVie, Alpine Immunology, AstraZeneca, BMS, Eli Lilly, GSK, Hi Bio, Janssen, Novartis, Pfizer, Roche, and Sandoz, Andrea Rubbert-Roth AbbVie, Amgen, BMS, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB, AbbVie, Amgen, BMS, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB, Yoshiya Tanaka AbbVie, Asahi-Kasei, Astellas, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, GSK, Janssen, Mitsubishi-Tanabe, Novartis, Pfizer, Sanofi, and YL Biologics, AbbVie, Asahi-Kasei, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, and Takeda, Kevin L. Winthrop AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, Roche, and UCB Pharma, AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, Roche, and UCB Pharma, Priscila Nakasato Employee of AbbVie and may hold stock or options, Derek Coombs Employee of AbbVie and may hold stock or options., Andrew Gara Employee of AbbVie and may hold stock or options., Nasser Khan Employee of AbbVie and may hold stock or options, Birgit Kovacs Employee of AbbVie and may hold stock or options, Sebastian Meerwein Employee of AbbVie and may hold stock or options, Jeffrey R. Curtis AbbVie, Amgen, Bristol Myers Squibb, CorEvitas, Janssen, Labcorp, Lilly, Novartis, Pfizer, Sanofi/Regeneron, and UCB, AbbVie, Amgen, Bristol Myers Squibb, CorEvitas, Janssen, Labcorp, Lilly, Novartis, Pfizer, Sanofi/Regeneron, and UCB.